Abstract
Carbon is unique in the variety of configurations it can adopt with itself and other elements. Here we show how ion beams can be used to nanostructure various diamond polytypes, epitaxially aligning them to a silicon substrate. The ready controllability of ion beams, which are already used to manufacture submicrometre-scale devices, means that our findings should enable new carbon and non-carbon materials to be nanostructured for a host of applications.
Similar content being viewed by others
Main
Metastable phases (including carbon) can be deposited using hyperthermal species (10–100 eV) by a 'shallow-implantation' process1. The energy barrier for their formation is overcome by trapping energetic species in subsurface positions of a 'mould' host matrix. Examples include amorphous, diamond-like carbon films with a local tetrahedral configuration2; transformation of carbon nanotubes to cubic diamond by electron bombardment3; and ion-beam nanostructuring of multiwalled, small-diameter carbon nanotubes4.
Diamond polytypes are formed by changing the stacking sequence of the closed-packed plane of the diamond crystal. We used ion beams to form diamond poly types (cubic, 3C; hexagonal, 2H; and rhombohedral, 9R, where the number denotes the number of closed-packed planes in a unit cell5) differing in the stacking order and periodicity of their closed-packed planes. The stacking sequence is controlled by the mould provided by the nucleation site1,6 and by the deposition conditions.
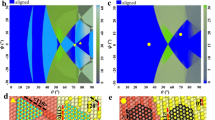
Films were prepared using a (CH4 or C2H2)/Ar/H2-fed Kaufmann source to bombard a Si(100) sample held at 600 or 700 °C with ions at 80 or 200 eV. The Fourier transform diffractogram of a cubic diamond crystal nucleated on a silicon step indicates the high-quality cubic-to-cubic epitaxial relationship between the Si and diamond, with a (111) diamond/Si interface and the absence of misorientation (Fig. 1a).
a, Cubic-diamond crystallite grown epitaxially on a silicon step; inset, crystallite diffractogram (Fourier transform). There is no misorientation between the diamond and silicon. b, Top left, 6-nm 9R diamond crystallite grown epitaxially on a Si step; bottom, part of a 60-nm 9R crystallite; top right, filtered image of 9R diamond showing the 9R repeat 'ABCBCACAB' sequence. c, Another diamond crystallite, also grown epitaxially on a Si step. Note the different symmetry of the 2H crystallite (right) and the sharp boundary with the 9R crystallite (left).
Figure 1b shows an epitaxial crystal and part of a similar large crystal, with its filtered image: the 9R registry is evident. Orientation relations for this phase are: [0001]9RDia//[-111]Si, [10-10]9RDia//[110]Si, [01-10]9RDia//[0-11]Si (hexagonal indexing).
The left part of a third crystallite (Fig. 1c) is 9R diamond, but its right part has a different stacking sequence and symmetry from 9R and cubic diamond (Fig. 1a, b). The lower-right portion of this crystallite gives a complex image (Moiré fringes, resulting from interference between beams diffracted from both the silicon and the diamond lattices). The upper-right part shows a grid of lines normal to each other, in contrast to the 109.5° angle seen in the high-resolution transmission electron micrographs of cubic diamond (Fig. 1a) and silicon (Fig. 1a, c). Comparison with image simulations for possible diamond polytypes indicates that this crystallite is a hexagonal polytype; the nucleation on a silicon step and the strong epitaxial relations (the same as those of the 9R polytype) are evident. Although these crystallites may contain defects, we have shown that different diamond polytypes can be structured, sometimes by sharp transition from one polytype to another (Fig. 1c).
Polytypes have similar properties with variations that may be useful7. Heterojunctions are an example of an application that can involve different polytypes7. Our findings indicate that metastable phases can be manipulated by using low-energy ion beams, offering new opportunities to control material synthesis.
References
Lifshitz, Y., Kasi, S. R. & Rabalais, J. W. Phys. Rev. Lett. 62, 1290–1293 (1989).
Lifshitz, Y. Diamond Rel. Mater. 8, 1659–1676 (1999).
Banhart, F. & Ajayan, P. M. Nature 382, 433–435 (1996).
Peng, H. Y. et al. Appl. Phys. Lett. 77, 2831–2833 (2000).
Ramsdell, R. S. Am. Mineral. 32, 64–82 (1947).
Lee, S. T. et al. Science 287, 104–106 (2000).
Morkoc, H. et al. J. Appl. Phys. 76, 1363–1398 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lifshitz, Y., Duan, X., Shang, N. et al. Epitaxial diamond polytypes on silicon. Nature 412, 404 (2001). https://doi.org/10.1038/35086656
Issue Date:
DOI: https://doi.org/10.1038/35086656
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.