Abstract
Ozone is a secondary pollutant and greenhouse gas that is formed from molecular oxygen in the presence of sunlight and nitrogen oxides. The extent of production also depends on the presence of volatile hydrocarbons, carbon monoxide and methane1,2,3,4,5,6. But we have discovered a surprising source of ozone which is generated in spontaneous bursts even in the absence of sunlight and nitrogen oxides — namely, the exuberant mass of colour-emitting sparklers that are lit during the Diwali festivities, which take place every year during October and November in Delhi, India. The underlying process of ozone formation resembles that induced by ultraviolet radiation in the stratosphere7,8.
Similar content being viewed by others
Main
We undertook a routine, real-time monitoring of the concentrations of NOx (NO and NO2), O3 and other microclimatic factors at a known pollutant-receptor site9 in Delhi in order to determine the effects of burning unprecedented numbers of fireworks on the local environment during the festive period in November 1999. We found that during the festival period the ozone concentration peaked at around noon and fell to negligible levels after sunset.
On Diwali night (7 November), a small build-up of O3 (9 ± 1 parts per billion by volume) was detected between 20:40 and 02:30 hours (results not shown). During this period, no correlation was found between NOx concentration and O3 formation, indicating that the ozone was unlikely to have been generated in reactions involving ambient NOx. This observation was surprisingly different from night-time O3 measurements obtained on the other dates, when no O3 formation was detected.
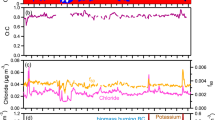
Further experiments carried out under different climatic conditions showed that there is a linear regression between the total amount of inflammable material present in sparklers and the cumulative O3 formed (correlation, 0.993). There was no change in ambient NOx concentration before, during or after these experiments (Fig. 1).
a–d, Values correspond to the simultaneous burning of one, three, four and six sparklers, respectively; vertical black lines indicate the duration of burning for the sparklers (starting at 0 s). O3 begins to accumulate in each case at around 100 s; this time lag corresponds to the volume of air in the assembly of the Teflon tube and manifold attached to the O3 and NO x analysers. During the experiments, the open end of the Teflon tube leading to the analysers was kept 900 cm away from the sparklers. The average weight of inflammable material (initial weight minus weight after burning) was 9.15 ± 0.85 g per sparkler. The burn time varied from 26 s for one sparkler to a maximum of 42 s for six; this variation was due to differences in the weight of the burning mixture. O3 formation was independent of microclimatic factors such as temperature and humidity of the ambient air. The concentrations of NO and NO2 did not change during the O3 burst. Further details are available from the authors.
Sparklers depend on a combination of different metal salts to generate their colour and sparkle10 — these include potassium perchlorate, sulphur, strontium nitrate, barium nitrate, sodium oxalate, calomel, aluminium and manganese. When burnt, a significant proportion of the light emitted by these constituents has a wavelength below 240 nm (ref. 11). The radiative energy of these emissions is sufficient to dissociate atmospheric molecular oxygen into atomic oxygen, enabling the reaction O2 + O → O3 to take place. This proposed mechanism could explain the formation of bursts of O3 without the participation of NOx, and is therefore similar to the process of ultraviolet-radiation-induced formation of O3 in the stratosphere7,8.
References
Ramanathan, V., Singh, H. B., Ciceron, R. J. & Kiehl, J. T. J. Geophys. Res. 90, 5547–5566 (1985).
Lacis, A. A., Weubbles, D. J. & Logan, J. A. J. Geophys. Res. 95, 9971–9981 (1990).
Wang, W. C. & Sze, N. D. Nature 286, 589–590 (1980).
Crutzen, P. J. & Zimmermann, P. H. Tellus 43A–B, 136–151(1991).
Walcek, C. J. & Yuan, H.-H J. Appl. Meteorol. 34, 1056–1069 (1995).
Johnson, D. W. et al. Nature 353, 617–621 (1991).
Chapman, S. R. Meteorol. Soc. Mem. 3, 103–125 (1930).
Brausser, G. P. et al. J. Geophys. Res. 95, 5639–5655 (1990).
Singh, A. et al. Atmos. Environ. 31, 3421–3427 (1997).
Holmes, H. H. in The Encyclopedia Americana Int. edn, Vol. 11, 263–265 (American Corp., New York, 1983).
Reader, J. & Corliss, C. H. in CRC Handbook of Chemistry and Physics 63rd edn (eds Weast, R. C. & Astle, M. J.) E205–E368 (CRC, Florida, 1982–83).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attri, A., Kumar, U. & Jain, V. Formation of ozone by fireworks. Nature 411, 1015 (2001). https://doi.org/10.1038/35082634
Issue Date:
DOI: https://doi.org/10.1038/35082634
This article is cited by
-
Multi-time-scale surface ozone exposure and associated premature mortalities over Indian cities in different climatological sub-regions
Air Quality, Atmosphere & Health (2024)
-
Dynamic changes in the characteristics of fine particles and their oxidative potential in the city of Taj (Agra, India): the untold story of fireworks display
Air Quality, Atmosphere & Health (2023)
-
Effects of Residential Customs on Spatio-Temporal Pollution Characteristics of Fireworks Burning during Chinese New Year
Asia-Pacific Journal of Atmospheric Sciences (2022)
-
Air pollution in five Indian megacities during the Christmas and New Year celebration amidst COVID-19 pandemic
Stochastic Environmental Research and Risk Assessment (2022)
-
Festival Ushered Pollutants in Indian Metropolitan Cities: Resemblance, Variance, and Concerns
Environmental Processes (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.