Abstract
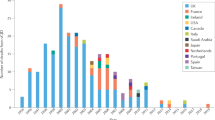
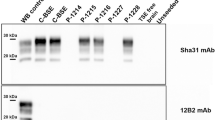
The systematic testing of slaughtered cattle aged over 30 months, or alternatively their elimination from the food chain, is an important component of a package of measures introduced in the European Union on 1 January 2001 to combat bovine spongiform encephalopathy (BSE) and protect human health. Here we explore the analytical limit of a rapid test designed to detect the abnormal prion protein associated with BSE in bovine brain and find that it is comparable to the limit of detection of infectivity in the conventional mouse bioassay1, which is impractical for systematic screening. The sensitivity of the biochemical test allows it to be used as a viable alternative to the destruction of all carcasses of cattle slaughtered after 30 months of age. Additional work is required to compare this analytical sensitivity with the diagnostic sensitivity of the test under conditions of routine post-mortem BSE diagnosis and surveillance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
European Commission in Oral Exposure of Humans to the BSE Agent: Infective Dose and Species Barrier 31 (Scientific Steering Committee meeting, 13–14 April 2000).
Moynagh, J. & Schimmel, H. Nature 400, 105 (1999).
European Commission The Evaluation of Tests for the Diagnosis of Transmissible Spongiform Encephalopathy in Bovines (http://europa.eu.int/comm/food/fs/bse/bse12_en.html).
Grassi, J. et al. Arch. Virol. 16 (suppl.), 197–205 (2000).
Wells, G. A. H. et al. Vet. Rec. 142, 103–106 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deslys, J., Comoy, E., Hawkins, S. et al. Screening slaughtered cattle for BSE. Nature 409, 476–478 (2001). https://doi.org/10.1038/35054134
Issue Date:
DOI: https://doi.org/10.1038/35054134
This article is cited by
-
Absolute Quantification of Prion Protein (90–231) Using Stable Isotope-Labeled Chymotryptic Peptide Standards in a LC-MRM AQUA Workflow
Journal of the American Society for Mass Spectrometry (2012)
-
Biochemical identification of bovine spongiform encephalopathies in cattle
Acta Neuropathologica (2007)
-
Properties of a disease-specific prion probe
Nature Medicine (2004)
-
Diagnosing prion diseases: needs, challenges and hopes
Nature Reviews Microbiology (2004)
-
Bovine Spongiform Encephalopathy (BSE) – Infectious, Contagious, Zoonotic or Production Disease?
Acta Veterinaria Scandinavica (2003)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.