Abstract
The g factor refers to the substantial overlap that exists between individual differences in diverse cognitive processes in humans. In this article, I argue that a mouse model of g could provide a powerful analytic tool for exploring cognitive processes that are linked functionally by genes.
Key Points
Summary
-
Cognitive neuroscience focuses on species-universal processes rather than on differences between individuals. Heredity is based on the naturally occurring genetic variation that underlies individual differences. For complex traits, many genes contribute to heritable variation. Common disorders may be the quantitative extreme of the same genetic factors that contribute to variability throughout the distribution of individual differences.
-
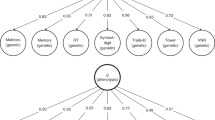
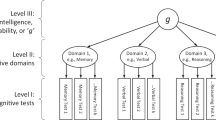
One of the most consistent findings from individual-variability research on human cognitive abilities and disabilities is that very diverse processes such as general reasoning, spatial ability and vocabulary highly interrelate. A technique called factor analysis best captures this overlap and yields a g factor that accounts for about 40% of the variance of diverse cognitive tests. The existence of g does not imply that a single physical, physiological or psychological process is responsible for g.
-
There are more studies addressing the genetics of human g than any other trait. These studies consistently converge on the conclusion that genetic factors contribute substantially to g. Multivariate genetic analysis indicates that what is common between cognitive abilities is genetic in origin whereas what is specific to each cognitive ability is largely environmental.
-
An animal model of g is needed to apply sophisticated neurobiological techniques to understand the brain mechanisms that mediate genetic influences on g. Most of the research on individual differences and genetics in learning and memory has used mice than all other non-human species combined, and the general acceptance of g in man rekindled interest in g in rodents. Evidence for g has emerged from several studies of diverse cognitive tasks in mice.
-
More research is needed to prove that g in mice is the same as g in man. Investigating g in mice requires measures that are reliable at the level of the individual mouse, large samples, and a battery of diverse cognitive measures. The strongest genetic evidence for congruence of g in mouse and man will come from identifying genes that are associated with g in both mouse and man. The strongest neurobiological evidence for congruence will come from showing congruence of brain function across species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gazzaniga, M. S. (ed.) Cognitive Neuroscience: A Reader (Blackwell, Oxford, 2000).
Thompson, R. F. The Brain: A Neuroscience Primer 3rd edn (Worth, New York, 2000).
Plomin, R., DeFries, J. C., McClearn, G. E. & McGuffin, P. Behavioral Genetics 4th edn (Worth, New York, 2001).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue . Nature 372, 425–432 (1994).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903– 908 (1997).
Guthrie, R. The introduction of newborn screening for phenylketonuria: A personal history . Eur. J. Pediatr. 155, S4– 5 (1996).
Plomin, R., Owen, M. J. & McGuffin, P. The genetic basis of complex human behaviors. Science 264, 1733–1739 ( 1994).
Gayán, J. et al. Quantitative-trait locus for specific language and reading deficits on chromosome 6p. Am. J. Hum. Genet. 64, 157–164 (1999).
Carroll, J. B. Human Cognitive Abilities (Cambridge Univ. Press, New York, 1993).
Anderson, M. Intelligence and Development: A Cognitive Theory (Blackwell, Oxford, 1992).
Baddeley, A. & Gathercole, S. in Learning and Individual Differences: Process, Trait, and Content Determinants (eds Ackerman, P. L., Kyllonen, P. C. & Roberts, R. D.) 31–50 (American Psychological Association, Washington DC, 1999).
Deary, I. M. Looking Down on Human Intelligence: From Psychometrics to the Brain (Oxford Univ. Press, Oxford, 2000).
Stauffer, J. M., Ree, M. J. & Carretta, T. R. Cognitive-components tests are not much more than g: an extension of Kyllonen's analyses. J. Gen. Psychol. 123, 193–205 (1996).
Spearman, C. 'General Intelligence' objectively determined and measured. Am. J. Psychol. 15, 201–293 (1904).
Jensen, A. R. The g Factor: The Science of Mental Ability (Praeger, Westport, Connecticut, 1998).
Deary, I. J., Whalley, L. J., Lemmon, H., Crawford, J. R. & Starr, J. M. The stability of individual differences in mental ability from childhood to old age: follow-up of the 1932 Scottish Mental Survey. Intelligence 28, 49– 55 (2000).
Gottfredson, L. S. Why g matters: The complexity of everyday life. Intelligence 24, 79–132 (1997).
Salthouse, T. A. & Czaja, S. J. Structural constraints on process explanations in cognitive aging. Psychol. Aging 15, 44–55 (2000).
Brody, N. Intelligence 2nd edn (Academic, New York, 1992).
Carroll, J. B. Reflections on Stephen Jay Gould's The Mismeasure of Man. Intelligence 21, 121–134 (1995).
Mackintosh, N. J. IQ and Human Intelligence (Oxford Univ. Press, Oxford, 1998).
Gould, S. J. The Mismeasure of Man 2nd edn (W. W. Norton, New York, 1996).
Gardner, H. Frames of Mind: The Theory of Multiple Intelligences (Basic Books, New York, 1983).
Sternberg, R. J. Beyond IQ: A Triarchic Theory of Human Intelligence (Cambridge Univ. Press, Cambridge, 1985).
Sternberg, R. J. & Gardner, M. K. in A Model for Intelligence (ed. Eysenck, J. H.) 231–254 (Springer–Verlag, New York, 1982).
Fodor, J. A. The Modularity of Mind (MIT Press, Cambridge, Massachusetts, 1983).
Pinker, S. The Language Instinct (William Morrow, New York, 1994 ).
Bouchard, T. J. Jr & McGue, M. Familial studies of intelligence: A review. Science 212, 1055–1059 (1981).
Fisher, P. J. et al. DNA pooling identifies QTLs for general cognitive ability in children on chromosome 4. Hum. Mol. Genet. 8, 915–922 (1999).
Plomin, R. in Molecular Genetics of Human Personality (eds Benjamin, J., Ebstein, R. & Belmaker, R. H.) (American Psychiatric Press, New York, in the press).
Plomin, R. Genetics and general cognitive ability. Nature 402, C25–C29 (1999).
Detterman, D. K. in The Nature of Intelligence (eds Bock, G. R., Goode, J. A. & Webb, K.) 136–148 (Wiley, Chichester, Novartis Foundation Symposium 233, 2000).
Kosslyn, S. & Plomin, R. in Psychiatric Neuroimaging Research: Contemporary Strategies (eds Dougherty, D., Rauch, S. L. & Rosenbaum, J. F.) 491–515 (American Psychiatric, Washington DC, 2001).
Anderson, B. in The Nature of Intelligence (eds Bock, G. R., Goode, J. A. & Webb, K.) 79–95 (Wiley, Chichester, Novartis Foundation Symposium 233, 2000).
Matzel, L. D. & Gandhi, C. C. The tractable contribution of synapses and their component molecules to individual differences in learning . Behav. Brain Res. 110, 53– 66 (2000).
Chandra, S. B. C., Hosler, J. S. & Smith, B. H. Heritable variation for latent inhibition and its correlation with reversal learning in honeybees (Apis mellifera). J. Comp. Psychol. 114, 86–97 (2000).
Sprott, R. L. & Staats, J. Behavioral studies using genetically defined mice — a bibliography. Behav. Genet. 5, 27–82 (1975).
Bovet, D. in Genetics, Environment and Intelligence (ed. Oliverio, A.) 79– 92 (North–Holland, Amsterdam, 1977).
Owen, E. H., Logue, S. F., Rasmussen, D. L. & Wehner, J. M. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience 80, 1087–1099 (1997).
Coren, S. The Intelligence of Dogs (Bantam Books, New York, 1994 ).
Tolman, E. in 39th Yearbook of the National Society for Studies of Education 111–119 (Public School Publishing Co., Bloomington, Illinois, 1924).
Tryon, R. C. The inheritance of maze-learning ability in rats. J. Comp. Psychol. 4, 1–8 (1940 ).
Heron, W. T. The inheritance of maze learning ability in rats. J. Comp. Psychol. 19, 77–89 ( 1935).
Heron, W. T. The inheritance of brightness and dullness in maze learning ability in the rat. J. Genet. Psychol. 59, 41– 49 (1941).
Thompson, W. R. The inheritance and development of intelligence. Proc. Assoc. Res. Nervous Mental Disorders 33, 209–231 (1954).
Thorndike, R. L. Organization of behavior in the albino rat. Genet. Psychol. Monographs 17, 1–70 (1935 ).
Tryon, R. C. in Comparative Psychology (ed. Moss, F. A.) 330– 365 (Prentice Hall, New York, 1946).
Anderson, B. Evidence from the rat for a general factor that underlies cognitive performance and that relates to brain size: intelligence? Neurosci. Lett. 153, 98–102 (1993).
Locurto, C. & Durkin, J. Problem-solving and individual differences in mice (Mus musculus) using water reinforcement. J. Comp. Psychol. (in the press).
Locurto, C. & Scanlon, C. Individual differences and a spatial learning factor in two strains of mice (Mus musculus). J. Comp. Psychol. 112, 344–352 (1998).
Paya-Cano, J. L., Galsworthy, M., Stephenson, J., Plomin, R. Developing a mouse model for the functional investigation of cognitive abilities and disabilities. Am. J. Med. Genet. 96, 560 (2000).
Spearman, C. The Abilities of Man: Their Nature and Measurement 403 (Macmillan, New York, 1927).
Martin, N. G. & Eaves, L. J. The genetical analysis of covariance structure. Heredity 38, 79– 95 (1977).
Petrill, S. A. Molarity versus modularity of cognitive functioning? A behavioral genetic perspective. Curr. Dir. Psychol. Sci. 6, 96–99 (1997).
Talbot, C. J. et al. High-resolution mapping of quantitative trait loci in outbred mice. Nature Genet. 21, 305– 308 (1999).
Acknowledgements
M. Galsworthy, J. L. Paya-Cano and S. Monleon contributed to this essay and are collaborators in our ongoing research on g in HS mice funded in part by a grant from the US National Institute of Child Health and Human Development.
Author information
Authors and Affiliations
Related links
Glossary
- QUANTITATIVE TRAIT LOCI
-
(QTLs) Genes (loci) in multiple-gene systems in which each QTL contributes quantitatively to a continuous distribution, thus creating a quantitative trait.
- PRINCIPAL COMPONENTS ANALYSIS
-
A statistical technique that weights tests to create a dimension that best represents the intercorrelations of the items.
- MULTIVARIATE GENETIC ANALYSIS
-
A quantitative genetic technique that analyses the covariance between traits rather than the variance of each trait considered on its own.
- GENETIC CORRELATION
-
A statistic from multivariate genetic analysis that indicates the extent to which genetic effects on one trait are the same as genetic effects on another trait.
- HERITABILITY
-
A statistic that describes the extent to which individual differences in a population can be ascribed to genetic differences between individuals.
- INBRED STRAINS
-
Animals produced by mating brother to sister for at least 20 generations, which results in homozygous animals with two copies of the same allele (form of gene) at all loci.
- HEBB-WILLIAMS MAZE
-
Rectangular field with a start box a a goal box at opposite ends of the apparatus. Different configurations are obtained by placing barriers at different points of the field.
- OUTBRED LINES
-
Animals produced by crossing inbred strains, which increases heterozygous animals with different alleles at all loci.
Rights and permissions
About this article
Cite this article
Plomin, R. The genetics of G in human and mouse. Nat Rev Neurosci 2, 136–141 (2001). https://doi.org/10.1038/35053584
Published:
Issue Date:
DOI: https://doi.org/10.1038/35053584
This article is cited by
-
Individual consistency in the learning abilities of honey bees: cognitive specialization within sensory and reinforcement modalities
Animal Cognition (2023)
-
Cognitive performance is linked to group size and affects fitness in Australian magpies
Nature (2018)
-
Song learning and cognitive ability are not consistently related in a songbird
Animal Cognition (2017)
-
Cognitive test batteries in animal cognition research: evaluating the past, present and future of comparative psychometrics
Animal Cognition (2017)
-
Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT)
Molecular Psychiatry (2014)