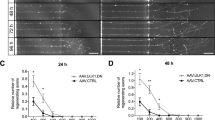
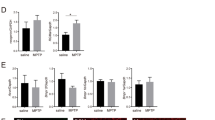
Abstract
Nogo has been identified as a component of the central nervous system (CNS) myelin that prevents axonal regeneration in the adult vertebrate CNS. Analysis of Nogo-A has shown that an axon-inhibiting domain of 66 amino acids is expressed at the extracellular surface and at the endoplasmic reticulum lumen of transfected cells and oligodendrocytes1. The acidic amino terminus of Nogo-A is detected at the cytosolic face of cellular membranes1 and may contribute to inhibition of axon regeneration at sites of oligodendrocyte injury2,3. Here we show that the extracellular domain of Nogo (Nogo-66) inhibits axonal extension, but does not alter non-neuronal cell morphology. In contrast, a multivalent form of the N terminus of Nogo-A affects the morphology of both neurons and other cell types. Here we identify a brain-specific, leucine-rich-repeat protein with high affinity for soluble Nogo-66. Cleavage of the Nogo-66 receptor and other glycophosphatidylinositol-linked proteins from axonal surfaces renders neurons insensitive to Nogo-66. Nogo-66 receptor expression is sufficient to impart Nogo-66 axonal inhibition to unresponsive neurons. Disruption of the interaction between Nogo-66 and its receptor provides the potential for enhanced recovery after human CNS injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
GrandPre, T., Nakamura, F., Vartanian, T. & Strittmatter, S. M. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature 403, 439–444 (2000).
Chen, M. S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
Prinjha, R. et al. Inhibitor of neurite outgrowth in humans. Nature 403, 383–384 ( 2000).
Schwab, M. & Caroni, P. Oligodendrocytes and CNS myelin are non-permissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 8, 2381– 2393 (1988).
Caroni, P. & Schwab, M. E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 106, 1281–1288 (1988).
Flanagan, J. G. & Leder, P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell 63, 185–194 ( 1990).
Schnell, L. & Schwab, M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343, 269– 272 (1990).
Buffo, A. et al. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured Purkinje cell axons. J. Neurosci. 20, 2275–2286 (2000).
Thallmair, M. et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature Neurosci. 1, 124–131 ( 1998).
Nakamura, F., Tanaka, M., Takahashi, T., Kalb, R. G. & Strittmatter, S. M. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron 21, 1093–1100 ( 1998).
Takahashi, T., Nakamura, F., Jin, Z., Kalb, R. G. & Strittmatter, S. M. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nature Neurosci. 1, 487–493 (1998).
Goshima, Y., Nakamura, F., Strittmatter, P. & Strittmatter, S. M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509– 514 (1995).
Huang, D. W., McKerracher, L., Braun, P. E. & David, S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron 24, 639– 647 (1999).
Acknowledgements
This work was supported by grants to S.M.S. from the NIH and the Christopher Reeve Paralysis Foundation. A.F. was an FCAR research fellow. S.M.S. is an Investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fournier, A., GrandPre, T. & Strittmatter, S. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration . Nature 409, 341–346 (2001). https://doi.org/10.1038/35053072
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35053072
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.