Abstract
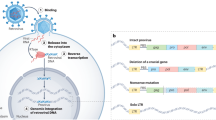
The high rate of recombination in retroviruses is due to the frequent template switching that occurs during reverse transcription. Although the mechanism that leads to this switch is still a matter of debate, there is increasing evidence that specific RNA structures are involved. And the implications might go beyond retroviral genetic variability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rein, A. Retroviral RNA packaging: a review. Arch. Virol. Suppl. 9, 513–522 (1994).
Gilboa, E., Mitra, S. W., Goff, S. & Baltimore, D. A detailed model of reverse transcription and tests of crucial aspects. Cell 18, 93–100 (1979).
Coffin, J. M., Hughes, S. H. & Varmus, H. E. Retroviruses (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1997).
Goodrich, D. W. & Duesberg, P. H. Retroviral recombination during reverse transcription. Proc. Natl Acad. Sci. USA 87, 2052–2056 (1990).
Hu, W. S. & Temin, H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc. Natl Acad. Sci. USA 87, 1556–1560 (1990).
Coffin, J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J. Gen. Virol. 42, 1–26 (1979).
Elder, J. H. et al. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc. Natl Acad. Sci. USA 74, 4676–4680 (1977).
Blair, D. G. Genetic recombination between avian leukosis and sarcoma viruses. Experimental variables and the frequencies of recombination. Virology 77, 534–544 (1977).
Sharp, P. M., Bailes, E., Robertson, D. L., Gao, F. & Hahn, B. H. Origins and evolution of AIDS viruses. Biol. Bull. 196, 338–342 (1999).
Peliska, J. A. & Benkovic, S. J. Mechanism of DNA strand transfer reactions catalysed by HIV-1 reverse transcriptase. Science 258, 1112–1118 (1992).
DeStefano, J. J., Mallaber, L. M., Rodriguez-Rodriguez, L., Fay, P. J. & Bambara, R. A. Requirements for strand transfer between internal regions of heteropolymer templates by human immunodeficiency virus reverse transcriptase. J. Virol. 66, 6370–6378 (1992).
DeStefano, J. J., Bambara, R. A. & Fay, P. J. The mechanism of human immunodeficiency virus reverse transcriptase-catalysed strand transfer from internal regions of heteropolymeric RNA templates. J. Biol. Chem. 269, 161–168 (1994).
Wu, W., Blumberg, B. M., Fay, P. J. & Bambara, R. A. Strand transfer mediated by human immunodeficiency virus reverse transcriptase in vitro is promoted by pausing and results in misincorporation. J. Biol. Chem. 270, 325–332 (1995).
Suo, Z. & Johnson, K. A. Effect of RNA secondary structure on the kinetics of DNA synthesis catalysed by HIV-1 reverse transcriptase. Biochemistry 36, 12459–12467 (1997).
Kim, J. K., Palaniappan, C., Wu, W., Fay, P. J. & Bambara, R. A. Evidence for a unique mechanism of strand transfer from the transactivation response region of HIV-1. J. Biol. Chem. 272, 16769–16777 (1997).
Darlix, J. L., Lapadat-Tapolsky, M., de Rocquigny, H. & Roques, B. P. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J. Mol. Biol. 254, 523–537 (1995).
Negroni, M. & Buc, H. Recombination during reverse transcription: an evaluation of the role of the nucleocapsid protein. J. Mol. Biol. 286, 15–31 (1999).
Guo, J., Henderson, L. E., Bess, J., Kane, B. & Levin, J. G. Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J. Virol. 71, 5178–5188 (1997).
Peliska, J. A., Balasubramanian, S., Giedroc, D. P. & Benkovic, S. J. Recombinant HIV-1 nucleocapsid protein accelerates HIV-1 reverse transcriptase catalysed DNA strand transfer reactions and modulates RNase H activity. Biochemistry 33, 13817–13823 (1994).
Rodriguez-Rodriguez, L., Tsuchihashi, Z., Fuentes, G. M., Bambara, R. A. & Fay, P. J. Influence of human immunodeficiency virus nucleocapsid protein on synthesis and strand transfer by the reverse transcriptase in vitro. J. Biol. Chem. 270, 15005–15011 (1995).
Allain, B., Lapadat-Tapolsky, M., Berlioz, C. & Darlix, J. -L. Transactivation of the minus-strand DNA transfer by nucleocapsid protein during reverse transcription of the retroviral genome. EMBO J. 13, 973–981 (1994).
Clodi, E., Semrad, K. & Schroeder, R. Assaying RNA chaperone activity in vivo using a novel RNA folding trap. EMBO J. 18, 3776–3782 (1999).
Tsuchihashi, Z. & Brown, P. DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J. Virol. 68, 5863–5870 (1994).
Rein, A., Henderson, L. E. & Levin, J. G. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem. Sci. 23, 297–301 (1998).
You, J. C. & McHenry, C. S. HIV nucleocapsid protein. J. Biol. Chem. 268, 16519–16527 (1993).
Negroni, M. & Buc, H. Copy-choice recombination by reverse transcriptases: reshuffling of genetic markers mediated by RNA chaperones. Proc. Natl Acad. Sci. USA 97, 6385–6390 (2000).
Jetzt, A. E. et al. High rate of recombination throughout the human immunodeficiency virus type 1 genome. J. Virol. 74, 1234–1240 (2000).
Suo, Z. & Johnson, K. A. RNA secondary structure switching during DNA synthesis catalysed by HIV-1 reverse transcriptase. Biochemistry 36, 14778–14785 (1997).
Schierup, M. H. & Hein, J. Consequences of recombination on traditional phylogenetic analysis. Genetics 156, 879–891 (2000).
Zhang, J. & Temin, H. M. Rate and mechanism of nonhomologous recombination during a single cycle of retroviral replication. Science 259, 234–238 (1993).
Chao, L. Fitness of RNA virus decreased by Muller's ratchet. Nature 348, 454–455 (1990).
Muller, H. J. The relation of recombination to mutational advance. Mutat. Res. 1, 2–9 (1964).
Xiong, Y. & Eickbush, T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 9, 3353–3362 (1990).
Coffin, J. M. in Fields Virology (eds Fields, B. N. et al.) 1437–1500 (Raven Press, New York, 1990).
Kohlstaedt, L. A., Wang, J., Friedman, J. M., Rice, P. A. & Steitz, T. A. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256, 1783–1790 (1992).
Furfine, E. S. & Reardon, J. E. Reverse transcriptase. RNase H from the human immunodeficiency virus. Relationship of the DNA polymerase and RNA hydrolysis activities. J. Biol. Chem. 266, 406–412 (1991).
Jacobo-Molina, A. et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc. Natl Acad. Sci. USA 90, 6320–6324 (1993).
Huang, H., Chopra, R., Verdine, G. L. & Harrison, S. C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282, 1669–1675 (1998).
Levin, H. L. It's prime time for reverse transcriptase. Cell 88, 5–8 (1997).
Acknowledgements
We acknowledge the French Agency for Research on AIDS (ANRS) for financial support. We are also indebted to M. Ricchetti for constant and helpful discussions, and to T. Heidmann for his help in elaborating concepts on the biology of retroelements.
Author information
Authors and Affiliations
Related links
Rights and permissions
About this article
Cite this article
Negroni, M., Buc, H. Retroviral recombination: what drives the switch?. Nat Rev Mol Cell Biol 2, 151–155 (2001). https://doi.org/10.1038/35052098
Issue Date:
DOI: https://doi.org/10.1038/35052098
This article is cited by
-
Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden
Genome Medicine (2017)
-
Influence of vector design and host cell on the mechanism of recombination and emergence of mutant subpopulations of replicating retroviral vectors
BMC Molecular Biology (2009)
-
Selective killing of HIV-1-positive macrophages and T cells by the Rev-dependent lentivirus carrying anthrolysin O from Bacillus anthracis
Retrovirology (2008)
-
A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action
Biology Direct (2006)