Abstract
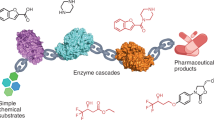
New catalytic synthetic methods in organic chemistry that satisfy increasingly stringent environmental constraints are in great demand by the pharmaceutical and chemical industries. In addition, novel catalytic procedures are necessary to produce the emerging classes of organic compounds that are becoming the targets of molecular and biomedical research. Enzyme-catalysed chemical transformations are now widely recognized as practical alternatives to traditional (non-biological) organic synthesis, and as convenient solutions to certain intractable synthetic problems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jones, J. B. Enzymes in organic synthesis. Tetrahedron 42, 3351–3403 (1986).
Sih, C. J. & Wu, S. H. Resolution of enantiomers via biocatalysis . Topics Stereochem. 19, 63– 125 (1989).
Wong, C.-H. & Whitesides, G. M. Enzymes in Synthetic Organic Chemistry (Pergamon, Oxford, 1994).
Drauz, K. & Waldmann, H. Enzyme Catalysis in Organic Synthesis (Wiley, Weinheim, 1995).
Wong, C.-H., Halcomb, R. L., Ichikawa, Y. & Kajimoto, T. Enzymes in organic synthesis: application to the problems of carbohydrate recognition. Parts 1 and 2. Angew. Chem. Int. Edn Engl. 34, 412–432; 521– 546 (1995).
Schoffers, E., Golebiowski, A. & Johnson, C. R. Enantioselective synthesis through enzymatic asymmetrization . Tetrahedron 52, 3769– 3826 (1996).
Faber, K. Biotransformations in Organic Chemistry 3rd edn (Springer, Berlin, 1997).
Klibanov, A. M. Why are enzymes less active in organic solvents than in water? Trends Biotechnol. 15, 97–101 (1997).
Schmid, R. D. & Verger, R. Lipases: interfacial enzymes with attractive applications. Angew. Chem. Int. Edn Engl. 37, 1608–1633 (1998).
Zaks, A. & Dodds, D. R. Biotransformations in the discovery and development of pharmaceuticals. Curr. Opin. Drug. Discov. Dev. 1, 290–303 ( 1998).
Roberts, S. M. Biocatalysis for Fine Chemicals Synthesis (Wiley, Weinheim, 1999).
Sugai, T. Application of enzyme- and microorganism-catalyzed reactions to organic synthesis . Curr. Org. Chem. 3, 373– 406 (1999).
Carrea, G. & Riva, S. Properties and synthetic applications of enzymes in organic solvents. Angew. Chem. Int. Edn Engl. 39, 2226–2254 (2000).
Machajewski, T. D. & Wong, C.-H. The catalytic asymmetric aldol reaction. Angew. Chem. Int. Edn Engl. 39, 1352–1374 (2000).
Fang, C.-M. & Wong, C.-H. Enzymes in organic synthesis: alteration of reversible reactions to irreversible processes. Synlett 6, 393–402 (1994).
Kazlauskas, R. J. Molecular modeling and biocatalysis: explanations, predictions, limitations, and opportunities. Curr. Opin. Chem. Biol. 4, 81–88 (2000).
Um, P.-J. & Drueckhammer, D. G. Dynamic enzymatic resolution of thioesters. J. Am. Chem. Soc. 120, 5605 –5610 (1998).
Persson, B. A., Larsson, A. L. E., Le Ray, M. & Backvall, J.-E. Ruthenium- and enzyme-catalyzed dynamic kinetic resolution of secondary alcohols . J. Am. Chem. Soc. 121, 1645– 1650 (1999).
Takayama, S., Lee, S. T., Hung, S.-C. & Wong, C.-H. Designing enzymatic resolution of amines. Chem. Commun. 127– 128 (1999).
Pathak, T. & Waldmann, H. Enzymes and protecting group chemistry . Curr. Opin. Chem. Biol. 2, 112– 120 (1998).
Bader, B. et al. Bioorganic synthesis of lipid-modified proteins for the study of signal transduction. Nature 403, 223– 226 (2000).
Kren, V. & Thiem, J. A multienzyme system for a one-pot synthesis of sialyl T-antigen. Angew. Chem. Int. Edn Engl. 34, 893–895 (1995).
Burkart, M. D., Izumi, M. & Wong, C.-H. Enzymatic regeneration of 3′-phosphoadenosine-5′-phosphosulfate using aryl sulfotransferase for the preparative enzymatic synthesis of sulfated carbohydrates. Angew. Chem. Int. Edn Engl. 38, 2747–2750 (1999).
DeLuca, C. et al. Enzymatic synthesis of hyaluronic acid with regeneration of sugar nucleotides. J. Am. Chem. Soc. 117, 5869–5870 (1995).
Gilbert, M. et al. The synthesis of sialylated oligosaccharides using a CMP-Neu5Ac synthetase/sialyltransferase fusion. Nature Biotechnol. 16, 769–772 (1998).
Wang, J.-Q. et al. Enhanced inhibition of human anti-gal antibody binding to mammalian cells by synthetic α-gal epitope polymers. J. Am. Chem. Soc. 121, 8174–8181 (1999).
Endo, T., Koizumi, S., Tabata, K. & Ozaki, A. Large-scale production of CMP-NeuAc and sialylated oligosaccharides through bacterial coupling. Appl. Microbiol. Biotechnol. 53, 257– 261 (2000).
Noguchi, T. & Shiba, T. Use of Escherichia coli polyphosphate kinase for oligosaccharide synthesis. Biosci. Biotechnol. Biochem. 62, 1594–1596 ( 1998).
Qian, X., Sujino, K., Otter, A., Palcic, M. M. & Hindsgaul, O. Chemoenzymatic synthesis of α(1,3)-Gal(NAc)-terminating glycosides of complex tertiary sugar alcohols. J. Am. Chem. Soc. 121, 12063–12072 ( 1999).
Nishida, Y., Tamakoshi, H., Kitagawa, Y., Kobayashi, K. & Thiem, J. A novel β-1,4-galactosyltransferase reaction to yield β-d-galactopyranosyl-(1-3)-linked disaccharides from l-sugars. Angew. Chem. Int. Edn Engl. 39, 2000–2003 (2000).
Tsuruta, O., Shinohara, G., Yuasa, H. & Hashimoto, H. UDP-N-acetyl-5-thio-galactosamine is a substrate of lactose synthase. Bioorg. Med. Chem. Lett. 7, 2523–2526 (1997).
Watt, G. M., Revers, L., Webberly, M. C., Wilson, I. B. H. & Flitsch, S. L. Efficient enzymatic synthesis of the core trisaccharide of N-glycans with a recombinant β-mannosyltransferase . Angew. Chem. Int. Edn Engl. 36, 2354– 2356 (1997).
Stein, A., Kula, M.-R. & Elling, L. Combined preparative enzymatic synthesis of dTDP-6-deoxy-4-keto- d-glucose from dTDP and sucrose. Glycoconjugate J. 15, 139–145 (1998).
Joo, H., Lin, Z. & Arnold, F. H. Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature 399, 670– 673 (1999).
Stewart, J. D. et al. Recombinant baker's yeast as a whole-cell catalyst for asymmetric Baeyer-Villiger oxidations. J. Am. Chem. Soc. 120, 3541–3548 (1998).
Burzlaff, N. I. et al. The reaction cycle of isopenicillin N-synthase observed by X-ray diffraction. Nature 401, 721– 724 (1999).
Schlichting, I. et al. The catalytic pathway of cytochrome P450cam at atomic resolution . Science 287, 1615–1622 (2000).
Elliott, S. J. et al. Regio- and stereoselectivity of particulate methane monooxygenase from Methylococcus capsulatus. J. Am. Chem. Soc. 119, 9949–9955 (1997).
Rosenzweig, A. C., Nordlund, P., Takahara, P. M., Frederick, C. A. & Lippard, S. J. Geometry of the soluble methane monooxygenase catalytic diiron center in two oxidation states. Chem. Biol. 2, 409–418 ( 1995).
Gavagan, J. E. et al. Glyoxylic acid production using microbial transformant catalysts . J. Org. Chem. 60, 3957– 3963 (1995).
Lakner, F. J., Cain, K. P. & Hager, L. P. Enantioselective epoxidation of ω-bromo-2-methyl-1-alkenes catalyzed by chloroperoxidase. Effect of chain length on selectivity and efficiency . J. Am. Chem. Soc. 119, 443– 444 (1999).
van Deurzen, M. P., van Rantwijk, F. & Sheldon R. A. Selective oxidation catalyzed by peroxidases. Tetrahedron 53, 13183–13220 (1997).
Margolia, A. L. Cross-linked enzyme crystals as novel materials for catalysis and chromatography . Chimia 50, 670–673 (1999).
Haring, D. & Schreier, P. Novel biocatalysts by chemical modification of known enzymes: cross-linked microcrystals of the semisynthetic peroxidase seleno-subtilisin. Angew. Chem. Int. Edn Engl. 37, 2471–2473 (1998).
Guo, Z.-w., Salamonczyk, G. M., Han, K., Machiya, K. & Sih, C. J. Enzymatic oxidative phenolic coupling . J. Org. Chem. 62, 6700– 6701 (1997).
Prigge, S. T., Kolhekar, A. S., Eipper, B. A., Mains, R. E. & Amzel, L. M. Amidation of bioactive peptides: the structure of peptidylglycine α-hydroxylating monooxygenase. Science 278, 1300–1305 ( 1997).
Hudlicky, T. et al. Toluene dioxygenase-mediated cis-hydroxylation of aromatics in enantioselective synthesis. Asymmetric total syntheses of pancratistatin and 7-deoxypancratistatin, promising antitumor agents. J. Am. Chem. Soc. 118, 10752–10765 ( 1996).
Liu, J.-Q., Kurihara, T., Miyagi, M., Esaki, N. & Soda, K. Reaction mechanism of l-2-haloacid dehalogenase of Pseudomonas sp. YL. J. Biol. Chem. 270, 18309– 18312 (1995).
Bordusa, F., Ullman, D., Elsner, C. & Jakubke, H.-D. Substrate mimetic mediated peptide synthesis: an irreversible ligation strategy that is independent of substrate specificity. Angew. Chem. Int. Edn Engl. 36, 2473–2475 (1997).
Plettner, E., DeSantis, G., Stabile, M. & Jones, J. B. Modulation of esterase and amidase activity of subtilisin Bacillus lentus by chemical modification of cysteine mutants. J. Am. Chem. Soc. 121, 4977–4981 ( 1999).
Witte, K., Seitz, O. & Wong, C.-H. Solution- and solid-phase synthesis of N-protected peptide esters of the benzyl type as substrates for subtilisin-catalyzed glycopeptide coupling. J. Am. Chem. Soc. 120, 1979– 1989 (1998).
Jackson, D. Y. et al. A designed peptide ligase for total synthesis of ribonuclease A with unnatural catalytic residues. Science 266, 243–247 (1994).
Kidd, R. D. et al. Breaking the low barrier hydrogen bond in a serine protease . Protein Sci. 8, 410–417 (1999).
Tolbert, T. J. & Wong, C.-H. Intein-mediated synthesis of proteins containing carbohydrates and other molecular probes . J. Am. Chem. Soc. 122, 5421– 5428 (2000).
Wang, L.-X. et al. Combined chemical and enzymatic synthesis of a C-glycopeptide and its inhibitory activity toward glycoamidases. J. Am. Chem. Soc. 119, 11137–11146 ( 1997).
Mackenzie, L. F., Wang, Q., Warren, R. A. J. & Withers, S. G. Glycosynthases: mutant glycosidases for oligosaccharide synthesis. J. Am. Chem. Soc. 120, 5583–5584 (1998).
Fort, S. et al. Highly efficient synthesis of β(1,4)-oligo- and polysaccharides using a mutant cellulase. J. Am. Chem. Soc. 122, 5429–5437 (2000).
Kobayashi, S., Kiyosada, T. & Shoda, S.-i. Synthesis of artificial chitin: irreversible catalytic behavior of a glycosyl hydrolase through a transition state analogue substrate . J. Am. Chem. Soc. 118, 13113– 13114 (1996).
Kimura, T., Takayama, S., Huang, H. & Wong, C.-H. A practical method for the synthesis of N-acetyl-d-lactosamine derivatives by the tandem use of galactose oxidase and β-galactosidase. Angew. Chem. Int. Edn Engl. 35, 2348– 2350 (1996).
Nishimura, S.-I. & Yamada, K. Transfer of ganglioside GM3 oligosaccharide from a water soluble polymer to ceramide by ceramide glycanase. A novel approach for the chemical-enzymatic synthesis of glycosphingolipids . J. Am. Chem. Soc. 119, 10555– 10556 (1997).
Zannetti, M. T., Walter, C., Knorst, M. & Fessner, W.-D. Fructose-1,6-bisphosphate aldolase from Staphylococcus carnosus: overexpression, structure prediction, stereoselectivity, and application in the synthesis of bicyclic sugars. Chem. Eur. J. 5, 1882–1890 (1999).
Shelton, M. C. et al. 2-Keto-3-deoxy-6-phosphogluconate aldolases as catalysts for stereocontrolled carbon-carbon bond formation. J. Am. Chem. Soc. 118, 2118–2125 ( 1996).
Miyazaki, T., Sato, H., Sakakibara, T. & Kajihara, Y. An approach to the precise chemoenzymatic synthesis of 13C-labeled sialyloligosaccharide on an intact glycoprotein: a novel one-pot [3-13C]-labeling method for sialic acid analogues by control of the reversible aldolase reaction, enzymatic synthesis of [3-13C]-NeuAc-α-(2,3)-[U-13C]-Gal-β-(1,4)-GlcNAc-β- sequence onto glycoprotein, and its conformational analysis by developed NMR techniques. J. Am. Chem. Soc. 122, 5678–5694 (2000).
Li, K. & Frost, J. W. Synthesis of vanillin from glucose . J. Am. Chem. Soc. 120, 10545– 10546 (1998).
Hoffmann, T. et al. Aldolase antibodies of remarkable scope. J. Am. Chem. Soc. 120, 2768–2779 (1998).
Arnold, F. H. Design by directed evolution. Acc. Chem. Res. 31, 125–131 (1998).
Reetz, M. T. & Jaeger, K.-E. Enantioselective enzymes for organic synthesis created by directed evolution. Chem. Eur. J. 6, 407–412 (2000).
Fong, S., Machajewski, T. D., Mak, C. C. & Wong, C.-H. Directed evolution of d-2-keto-3-deoxy-6-phosphogluconate aldolase to new variants for the efficient synthesis of d- and l-sugars . Chem. Biol. 7, 873–883 (2000).
Janda, K. D. et al. Chemical selection for catalysis in combinatorial antibody libraries. Science 275, 945– 948 (1997).
Jestin, J.-L., Kristensen, P. & Winter, G. A method for the selection of catalytic activity using phage display and proximity coupling. Angew. Chem. Int. Edn Engl. 38, 1124–1127 ( 1999).
Cane, D. E., Walsh, C. T. & Khosla, C. Harnessing the biosynthetic code: combinations, permutations, and mutations. Science 282, 63– 68 (1998).
Shimizu, S., Ogawa, J., Kataoka, M. & Kobayashi, M. Screening of novel microbial enzymes for the production of biologically and chemically useful compounds. Adv. Biochem. Eng. Biotechnol. 58 , 45–88 (1997).
Schoemaker, H. E. et al. Application of enzymes in industrial organic synthesis. Chimia 51, 308–310 ( 1997).
Patel, R. N. Stereoselective Biocatalysis (Dekker, New York, 2000 ).
Acknowledgements
We thank P. Sears for assistance with figure preparation, and other co-workers whose names are listed in the references. We also extend apologies to those whose biocatalysis research was not cited owing to space constraints. This research was supported by the NIH and NSF.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koeller, K., Wong, CH. Enzymes for chemical synthesis. Nature 409, 232–240 (2001). https://doi.org/10.1038/35051706
Issue Date:
DOI: https://doi.org/10.1038/35051706
This article is cited by
-
Citrus limetta peels derived carbon dots as highly active carbocatalyst for carbon–carbon bond formation
Clean Technologies and Environmental Policy (2023)
-
Single amino acid bionanozyme for environmental remediation
Nature Communications (2022)
-
Reaction pathway engineering converts a radical hydroxylase into a halogenase
Nature Chemical Biology (2022)
-
Techniques and support materials for enzyme immobilization using Ugi multicomponent reaction: an overview
Journal of the Iranian Chemical Society (2022)
-
Simultaneous purification and characterization of detergent-stable, solvent-tolerant haloextremozymes protease and lipase from Haloferax sp. strain GUBF 2
Archives of Microbiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.