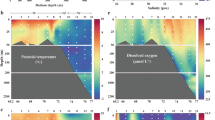
Abstract
Eutrophication of surface waters and hypoxia in bottom waters has been increasing in many coastal areas1,2,3,4, leading to very large depletions of marine life in the affected regions4. These areas of high surface productivity and low bottom-water oxygen concentration are caused by increasing runoff of nutrients from land. Although the local ecological and socio-economic effects have received much attention2,3,4, the potential contribution of increasing hypoxia to global-change phenomena is unknown. Here we report the intensification of one of the largest low-oxygen zones in the ocean, which develops naturally over the western Indian continental shelf during late summer and autumn. We also report the highest accumulations yet observed of hydrogen sulphide (H2S) and nitrous oxide (N2O) in open coastal waters. Increased N2O production is probably caused by the addition of anthropogenic nitrate and its subsequent denitrification, which is favoured by hypoxic conditions. We suggest that a global expansion of hypoxic zones may lead to an increase in marine production and emission of N2O, which, as a potent greenhouse gas, could contribute significantly to the accumulation of radiatively active trace gases in the atmosphere5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Turner, R. E. & Rabalais, N. N. Coastal eutrophication near the Mississippi River delta. Nature 368, 619–621 (1994).
Diaz, R. J. & Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33, 245 –303 (1995).
Rabalais, N. N. Oxygen depletion in coastal waters. [online] (cited 3/3/2000) 〈http://state_of_coast. noaa.gov/bulletins/html/hyp_09/hyp.html〉 (NOAA State of the Coast Report, National Oceanic and Atmospheric Administration (NOAA), Silver Spring, Maryland, 2000).
Malakoff, D. Death by suffocation in the Gulf of Mexico. Science 281, 190–192 (1998).
Houghton, J. T. et al. (eds) Climate Change 1995: The Science of Climate Change (Cambridge Univ. Press, Cambridge, 1996).
Banse, K. On upwelling and bottom-trawling off the southwest coast of India. J. Mar. Biol. Ass. India 1, 33–49 (1959).
Banse, K. Hydrography of the Arabian sea shelf of India and Pakistan and effects on demersal fishes. Deep-Sea Res. 15, 45– 79 (1968).
Shetye, S. R. et al. Hydrography and circulation off the west coast of India during the Southwest Monsoon 1987. J. Mar. Res. 48, 359–378 (1990).
Jayakumar, D. A., Naqvi, S. W. A., Narvekar, P. V. & George, M. D. Methane in coastal and offshore waters of the Arabian Sea. Mar. Chem. (in the press).
Calvert, S. E. & Price, N. B. Upwelling and nutrient regeneration in the Benguela Current, October, 1968. Deep-Sea Res. 18, 505–523 (1971).
Codispoti, L. A. & Packard, T. T. Denitrification rates in the eastern tropical South Pacific. J. Mar. Res. 38, 453–477 (1980).
Naqvi, S. W. A. Some aspects of the oxygen-deficient conditions and denitrification in the Arabian Sea. J. Mar. Res. 45, 1049– 1072 (1987).
Codispoti, L. A. et al. High nitrite levels off northern Peru: A signal of instability in the marine denitrification rate. Science 233, 1200–1202 (1986).
Carruthers, J. N., Gogate, S. S., Naidu, J. R. & Laevastu, T. Shoreward upslope of the layer of minimum oxygen off Bombay: Its influence on marine biology, especially fisheries. Nature 183 , 1084–1087 (1959).
Progress Report No. 3, 1– 13 (UNDP/FAO Pelagic Fishery Project, Bergen/Cochin, 1973).
Redfield, A. C., Ketchum, B. H. & Richards, F. A. in The Sea (ed. Hill, M. N.), Vol 2, 26–77 (Interscience, New York, 1963 ).
Codispoti, L. A. et al. in Oceanography of the Indian Ocean (ed. Desai, B. N.) 271–284 (Oxford-IBH, New Delhi, 1992 ).
Law, C. S & Owens, N. J. P. Significant flux of atmospheric nitrous oxide from the northwest Indian Ocean. Nature 346, 826–829 (1990).
Naqvi, S. W. A. & Noronha, R. J. Nitrous oxide in the Arabian Sea. Deep-Sea Res. 38, 871 –890 (1991).
Goreau, T. J. et al. Production of NO-2 and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 40, 526–532 (1980).
Yoh, M., Terai, H. & Saijo, Y. Accumulation of nitrous oxide in the oxygen deficient layer of freshwater lakes. Nature 301, 327 –329 (1983).
Firestone, M. K. & Tiedje, J. M. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl. Environ. Microbiol. 38, 673– 679 (1979).
Liss, P. & Merlivat, L. in The Role Of Air-Sea Exchange In Geochemical Cycling (ed. Buat-Menart, P.) 113– 128 (Riedel, Dordrecht, 1986).
Wanninkhof, R. H. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. 97, 7373–7382 (1992).
Naqvi, S. W. A. et al. Budgetary and biogeochemical implications of N 2O isotope signatures in the Arabian Sea. Nature 394, 462–464 (1998).
Bange, H. W. et al. A revised nitrogen budget for the Arabian Sea. Global Biogeochem. Cycles (in the press).
Kitoh, A., Yukimoto, S., Noda, A. & Motoi, T. Simulated changes in the Asian summer monsoon at times of increased atmospheric CO2. J. Meteorol. Soc. Japan 75, 1019– 1031 (1997).
Justić, D., Rabalais, N. N. & Turner, R. E. Effects of climate change on hypoxia in coastal waters: a doubled CO2 scenario for the northern Gulf of Mexico. Limnol. Oceanogr. 41, 992–1003 (1996).
Grasshoff, K., Ehrhardt, M. & Kremling, K. (eds) Methods of Seawater Analysis (Verlag Chemie, Weinheim, 1983).
Weiss, R. F. & Price, B. A. Nitrous oxide solubility in water and seawater. Mar. Chem. 8, 347– 359 (1980).
Acknowledgements
This work forms a part of the LOICZ-India programme of the Department of Ocean Development (DOD); we thank the Sagar Sampada and Sagar Kanya Management Cells of DOD for the generous allocation of ship time. The custom-made bags used for incubation experiments were given by B. Ward and J. Fernandes, B. Jose and D. Shenoy extended technical assistance at sea. Discussions with K. Banse, L. Codispoti and N. Rabalais greatly improved the content of the paper.
Author information
Authors and Affiliations
Supplementary information
Rights and permissions
About this article
Cite this article
Naqvi, S., Jayakumar, D., Narvekar, P. et al. Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature 408, 346–349 (2000). https://doi.org/10.1038/35042551
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35042551
This article is cited by
-
Contrasting patterns in pH variability in the Arabian Sea and Bay of Bengal
Environmental Science and Pollution Research (2024)
-
A study on microzooplankton community from the coastal waters of eastern Arabian Sea: emphasis on the dominance of heterotrophic dinoflagellates
Environmental Monitoring and Assessment (2023)
-
Impact of domestic and industrial effluent on marine environment at Karachi Port Trust (KPT) coastal area, Pakistan
Environmental Monitoring and Assessment (2023)
-
Spatio-Temporal Dynamics of Phytoplankton in the Mandovi Estuary, on the Central West Coast of India During Post Monsoon
Thalassas: An International Journal of Marine Sciences (2022)
-
Organic carbon dynamics in the continental shelf waters of the eastern Arabian Sea
Environmental Monitoring and Assessment (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.