Key Points
-
Iron balance must be strictly maintained to ensure that adequate amounts of iron are available for vital functions and to avoid the toxicity that results from iron excess. Disorders of iron deficiency and iron overload develop when iron balance is disrupted.
-
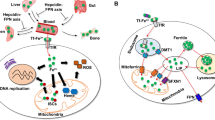
Iron is absorbed by enterocytes in the proximal intestine; it is stored in hepatocytes, used primarily by erythroid cells, and is recycled by specialized macrophages. There are similarities in the iron-transport strategies used by these different cell types, but tissue-specific differences also exist.
-
Two iron transporters, DMT1 (an importer) and ferroportin1 (an exporter — also known as Ireg1 or MTP1), have been identified by positional cloning.
-
Targeted mutagenesis has produced new mouse models of human iron disorders, including models for hereditary haemochromatosis and aceruloplasminaemia.
-
Despite recent advances in our understanding of iron absorption and iron recycling, many mechanistic details remain to be understood.
Abstract
Disorders that perturb iron balance are among the most prevalent human diseases, but until recently iron transport remained poorly understood. Over the past five years, genetic studies of patients with inherited iron homeostasis disorders and the analysis of mutant mice, rats and zebrafish have helped to identify several important iron-transport proteins. With information being mined from the genomes of four species, the study of iron metabolism has benefited enormously from positional-cloning efforts. Complementing the genomic strategy, targeted mutagenesis in mice has produced new models of human iron diseases. The animal models described in this review offer valuable tools for investigating iron homeostasis in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Finch, C. Regulators of iron balance in humans. Blood 84, 1697–1702 (1994).
Andrews, N. C. & Fleming, M. D. Commentary on: ferrokinetics in the syndrome of familial hypoferremic microcytic anemia with iron malabsorption . J. Pediatr. Hematol. Oncol. 21, 353– 355 (1999).
Buchanan, G. R. & Sheehan, R. G. Malabsorption and defective utilization of iron in three siblings. J. Pediatr. 98, 723–728 ( 1981).
Hartman, K. R. & Barker, J. A. Microcytic anemia with iron malabsorption: An inherited disorder of iron metabolism. Am. J. Hematol. 51, 269–275 (1996).
Pearson, H. A. & Lukens, J. N. Ferrokinetics in the syndrome of familial hypoferremic microcytic anemia with iron malabsorption. J. Pediatr. Hematol. Oncol. 21, 412– 417 (1999).
Schade, A. L. & Caroline, L. An iron-binding component in human blood plasma. Science 104, 340– 341 (1946).
Bannerman, R. M. Genetic defects of iron transport. Fed. Proc. 35, 2281 (1976).
Bernstein, S. E. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J. Lab. Clin. Med. 110, 690–705 (1987).
Huggenvik, J. I. et al. A splicing defect in the mouse transferrin gene leads to congenital atransferrinemia. Blood 74, 482– 486 (1989).
Trenor, C. C., Campagna, D. R., Sellers, V. M., Andrews, N. C. & Fleming, M. D. The molecular defect in hypotransferrinemic mice. Blood 96, 1113–1118 (2000).
Kaplan, J. et al. Regulation of the distribution of tissue iron. Lessons learned from the hypotransferrinemic mouse. Ann. NY Acad. Sci. 526, 124–135 (1988).
Simpson, R. J. et al. Tissue iron loading and histopathological changes in hypotransferrinaemic mice. J. Pathol. 171, 237– 244 (1993).
Heilmeyer, L. et al. Congenital transferrin deficiency in a seven-year old girl . German Med. Mon. 86, 1745– 1751 (1961).
Goya, N., Miyazaki, S., Kodate, S. & Ushio, B. A family of congenital atransferrinemia. Blood 40, 239– 245 (1972).
Hamill, R. L., Woods, J. C. & Cook, B. A. Congenital atransferrinemia: a case report and review of the literature. Am. J. Clin. Pathol. 96, 215–218 (1991).
Russell, E. S., McFarland, E. C. & Kent, E. L. Low viability, skin lesions, and reduced fertility associated with microcytic anemia in the mouse. Transpl. Proc. 2, 144–151 ( 1970).
Edwards, J. A. & Hoke, J. E. Defect of intestinal mucosal iron uptake in mice with hereditary microcytic anemia. Proc. Soc. Exp. Biol. Med. 141, 81– 84 (1972).
Riedel, H. D., Remus, A. J., Fitscher, B. A. & Stremmel, W. Characterization and partial purification of a ferrireductase from human duodenal microvillus membranes. Biochem. J. 309, 745–748 (1995).
Edwards, J. A. & Hoke, J. E. Red cell iron uptake in hereditary microcytic anemia. Blood 46, 381–388 (1975).
Harrison, D. E. Marrow transplantation and iron therapy in mouse hereditary microcytic anemia . Blood 40, 893–901 (1972).
Fleming, M. D. et al. Microcytic anemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genet. 16, 383–386 (1997).Provided the first evidence that Nramp2 (the gene encoding DMT1) is mutated in mk mice and is a major intestinal iron transporter.
Supek, F., Supekova, L., Nelson, H. & Nelson, N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl Acad. Sci. USA 93, 5105–5110 (1996).
Gunshin, H. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482– 488 (1997).DMT1 (termed DCT1 in this study) was isolated by expression cloning in Xenopus oocytes and shown to transport ferrous iron and other divalent metal ions.
Su, M. A., Trenor, C. C., Fleming, J. C., Fleming, M. D. & Andrews, N. C. The G185R mutation disrupts function of iron transporter Nramp2. Blood 92, 2157 –2163 (1998).
Canonne-Hergaux, F. et al. The NRAMP2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood (in the press).
Oates, P. S. & Morgan, E. H. Defective iron uptake by the duodenum of Belgrade rats fed diets of different iron contents. Am. J. Physiol. 270, G826–G832 ( 1996).
Sladic-Simic, D. et al. A thalassemia-like disorder in Belgrade laboratory rats. Ann. NY Acad. Sci. 165, 93–99 (1969).
Bowen, B. J. & Morgan, E. H. Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood 70, 38–44 (1987).
Edwards, J., Huebers, H., Kunzler, C. & Finch, C. Iron metabolism in the Belgrade rat. Blood 67, 623– 628 (1986).
Fleming, M. D. et al. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl Acad. Sci. USA 95, 1148–1153 ( 1998).By identifying a mutation in the b rat, DMT1 was shown to transport iron into and out of endosomes.
Canonne-Hergaux, F., Gruenheid, S., Ponka, P. & Gros, P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 93, 4406–4417 (1999).
Gruenheid, S. et al. The iron transport protein NRAMP2 is an integral membrane glycoprotein that co-localizes with transferrin in recycling endosomes. J. Exp. Med. 189, 831–841 (1999).
Farcich, E. A. & Morgan, E. H. Diminished iron acquisition by cells and tissues of Belgrade laboratory rats. Am. J. Physiol. 262, R220–R224 (1992).
Rodrigues, V., Cheah, P. Y., Ray, K. & Chia, W. malvolio, the Drosophila homologue of mouse NRAMP–1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behaviour. EMBO J. 14, 3007– 3020 (1995).
Orgad, S., Nelson, H., Segal, D. & Nelson, N. Metal ions suppress the abnormal taste behaviour of the Drosophila mutant malvolio. J. Exp. Biol. 201, 115–120 (1998).
Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 ( 1996).
Kingston, P. J., Bannerman, C. E. & Bannerman, R. M. Iron deficiency anaemia in newborn sla mice: a genetic defect of placental iron transport. Br. J. Haematol. 40, 265–276 ( 1978).
Edwards, J. A. & Bannerman, R. M. Hereditary defect of intestinal iron transport in mice with sex-linked anemia. J. Clin. Invest. 49, 1869–1871 (1970).
Pinkerton, P. H., Bannerman, R. M., Doeblin, T. D., Benisch, B. M. & Edwards, J. A. Iron metabolism and absorption studies in the X-linked anaemia of mice. Br. J. Haematol. 18, 211–228 (1970).
Vulpe, C. D. et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nature Genet. 21, 195–199 ( 1999).Showed that hephaestin is mutated in sla mice and is involved in intestinal iron transport.
Donovan, A. et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 (2000).Reported the positional cloning of the first vertebrate iron exporter, ferroportin1/IREG1/MTP1.
McKie, A. T. et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 ( 2000).In this study, ferroportin1/IREG1/MTP1 was isolated by differential expression analysis.
Abboud, S. & Haile, D. J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 ( 2000).
Harrison, P. M. & Arosio, P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1275, 161–203 (1996).
Cossee, M. et al. Inactivation of the friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219–1226 ( 2000).
Ferreira, C. et al. Early embryonic lethality of H ferritin gene deletion in mice . J. Biol. Chem. 275, 3021– 3024 (2000).
Feder, J. N. et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genet. 13, 399–408 (1996).The HFE gene (originally designated HLA-H ) is reported to be mutated in most patients with hereditary haemochromatosis.
Lebron, J. A. et al. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell 93, 111–123 (1998).
Parkkila, S. et al. Association of the transferrin receptor in human placenta with HFE, the protein defective in hereditary haemochromatosis. Proc. Natl Acad. Sci. USA 94, 13198– 13202 (1997).
Bennett, M. J., Lebron, J. A. & Bjorkman, P. J. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature 403, 46–53 (2000).
Bahram, S. et al. Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc. Natl Acad. Sci. USA 96, 13312–13317 (1999).
Levy, J. E., Montross, L. K., Cohen, D. E., Fleming, M. D. & Andrews, N. C. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood 94, 9–11 (1999). Mice lacking HFE have more severe iron loading than mice carrying the mutation found in human patients with haemochromatosis.
Zhou, X. Y. et al. HFE gene knockout produces mouse model of hereditary hemochromatosis . Proc. Natl Acad. Sci. USA 95, 2492– 2497 (1998).Hfe knockout mice have iron overload, similar to human patients with haemochromatosis.
Levy, J. E., Montross, L. K. & Andrews, N. C. Genes that modify the hemochromatosis phenotype in mice. J. Clin. Invest. 105, 1209– 1216 (2000).Compound mutant mice, carrying mutations in Hfe and in genes involved in iron transport, show that iron overload in haemochromatosis occurs through the same transport pathway as normal iron uptake.
Cartwright, G. E., Gubler, C. J., Bush, J. A. & Wintrobe, M. M. Studies on copper metabolism. XVII. Further observations on the anemia of copper deficiency in swine. Blood 11, 143 (1956).
Lee, G. R., Nacht, S., Lukens, J. N. & Cartwright, G. E. Iron metabolism in copper-deficient swine. J. Clin. Invest. 47, 2058–2069 (1968).
Osaki, S. & Johnson, D. A. Mobilization of liver iron by ferroxidase (ceruloplasmin). J. Biol. Chem. 244, 5757–5758 (1969).
Osaki, S., Johnson, D. A. & Frieden, E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J. Biol. Chem. 246, 3018–3023 (1971).
Harris, Z. L., Klomp, L. W. & Gitlin, J. D. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am. J. Clin. Nutr. 67, S972–S977 ( 1998).
Harris, Z. L. et al. Aceruloplasminemia: Molecular characterization of this disorder of iron metabolism. Proc. Natl Acad. Sci. USA 92, 2539–2543 (1995).
Morita, H. et al. Hereditary ceruloplasmin deficiency with hemosiderosis: a clinicopathological study of a Japanese family. Ann. Neurol. 37, 646–656 (1995).
Yoshida, K. et al. A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nature Genet. 9, 267–272 (1995).
Harris, Z. L., Durley, A. P., Man, T. K. & Gitlin, J. D. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl Acad. Sci. USA 96, 10812–10817 (1999). This targeted disruption produced a mouse model for aceruloplasminaemia, and showed that ceruloplasmin is important for iron efflux from cells.
Levy, J. E., Jin, O., Fujiwara, Y., Kuo, F. & Andrews, N. C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nature Genet. 21, 396–399 (1999). This targeted disruption of the transferrin receptor gene showed it to be essential for erythropoiesis but not for the early development of most other tissues.
Kawabata, H. et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J. Biol. Chem. 274, 20826–20832 (1999).
Camaschella, C. et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nature Genet. 25, 14– 15 (2000).This paper reported that a homologue of the transferrin receptor, TFR2, is mutated in some patients with non-HFE haemochromatosis.
Olynyk, J. K. et al. A population-based study of the clinical expression of the hemochromatosis gene. N. Engl. J. Med. 341, 718–724 (1999).
Mura, C., Raguenes, O. & Ferec, C. HFE mutations analysis in 711 hemochromatosis probands: evidence for S65C implication in mild form of hemochromatosis. Blood 93, 2502–2505 ( 1999).
Gordeuk, V. et al. Iron overload in Africa. Interaction between a gene and dietary iron content. N. Engl. J. Med. 326, 95– 100 (1992).
Moyo, V. M. et al. Traditional beer consumption and the iron status of spouse pairs from a rural community in Zimbabwe. Blood 89, 2159–2166 (1997).
Camaschella, C. et al. Juvenile and adult hemochromatosis are distinct genetic disorders . Eur. J. Hum. Genet. 5, 371– 375 (1997).
Roetto, A. et al. Juvenile hemochromatosis locus maps to chromosome 1q. Am. J. Hum. Genet. 64, 1388–1393 (1999).
Pietrangelo, A. et al. Hereditary hemochromatosis in adults without pathogenic mutations in the hemochromatosis gene. N. Engl. J. Med. 341, 725–732 (1999).
Cox, T. C. et al. X-linked pyridoxine-responsive sideroblastic anemia due to a Thr388-to-Ser substitution in erythroid 5-aminolevulinate synthase. N. Engl. J. Med. 330, 675–679 (1994).
Beris, P. et al. Iron overload in patients with sideroblastic anaemia is not related to the presence of the haemochromatosis Cys282Tyr and His63Asp mutations. Br. J. Haematol. 104, 97–99 (1999).
Chiu, M. K. & Davey, A. M. Neonatal hemochromatosis. Clin. Pediatr. 36, 607–610 (1997).
Edwards, C. Q. et al. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N. Engl. J. Med. 318, 1355 –1362 (1988).
Simon, M., Bourel, M., Fauchet, R. & Genetet, B. Association of HLA-A3 and HLA-B14 antigens with idiopathic haemochromatosis. Gut 17, 332–334 ( 1976).
Pandolfo, M. Molecular pathogenesis of Friedreich ataxia. Arch. Neurol. 56, 1201–1208 (1999).
Acknowledgements
I am grateful to all of the members of my laboratory for sharing their insights into iron metabolism. I appreciate help from Bernard Mathey-Prevot, Renee Ned, Carolyn Pettibone and Mark Fleming in providing criticism on an earlier version of the manuscript. Our studies are supported by the National Institutes of Health. I am an Associate Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Supplementary information
Related links
Related links
DATABASE LINKS
FURTHER INFORMATION
Major histocompatibility complex
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- HAEM
-
Haem proteins contain an iron complex of porphyrin, usually protoporphyrin IX, and function as catalysts in many biological processes.
- REDOX ACTIVITY
-
Oxidation and reduction activity, involving movement of electrons between different chemical entities.
- CYTOCHROMES
-
Haemoproteins that take advantage of valence changes in haem iron to facilitate electron or hydrogen transport.
- ERYTHROPOIESIS
-
The production of red blood cells. Erythropoiesis takes place in the bone marrow (humans and mice) and spleen (mice only).
- PICA
-
Pica is the compulsive consumption of non-nutritive substances including paint chips, salt, ice and clay.
- SIDEROBLASTIC ANAEMIA
-
Anaemia characterized by the presence of stainable iron granules in the cytoplasm of erythroid precursors (erythroblasts).
- ACERULOPLASMINAEMIA
-
Absence of serum ceruloplasmin. An autosomal, recessive disorder, leading to neurodegenerative disease, liver iron overload and diabetes, caused by mutations in the ceruloplasmin gene.
- TRANSFERRIN
-
An abundant plasma glycoprotein that binds iron with high affinity.
- SYNCYTIOTROPHOBLAST
-
Part of the placenta; the syncytial outer layer of the trophoblast, through which the embryo receives nutrients from the mother.
- ENTEROCYTES
-
Absorptive cells lining the intestine.
- HEPATOCYTES
-
The parenchymal cells of the liver.
- HOLOTRANSFERRIN
-
Transferrin loaded with iron.
- APOTRANSFERRIN
-
Transferrin that does not contain bound iron.
- MICROCYTIC
-
When red blood cells are smaller than normal, typically because of defects in haemoglobin production.
- HYPOCHROMIC
-
When red blood cells are poorly haemoglobinized, typically because of defects in haemoglobin production.
- ATRANSFERRINAEMIA
-
Absence of serum transferrin. An autosomal recessive disorder, associated with severe microcytic, hypochromic anaemia and tissue iron overload. Also called hypotransferrinaemia.
- RETICULOCYTES
-
The youngest red blood cells normally found in the circulation, freshly released from the bone marrow (or other site of erythropoiesis).
- NON-HAEM IRON
-
Iron from sources other than haem proteins.
- SIDEROSIS
-
A general term for iron overload.
- PHLEBOTOMY
-
Deliberate removal of venous blood from a patient.
- HYDROPS
-
Swelling caused by the accumulation of fluid in tissues and the body cavity, which often occurs as a result of severe anaemia.
Rights and permissions
About this article
Cite this article
Andrews, N. Iron homeostasis: insights from genetics and animal models. Nat Rev Genet 1, 208–217 (2000). https://doi.org/10.1038/35042073
Issue Date:
DOI: https://doi.org/10.1038/35042073
This article is cited by
-
ZIF-8 coated gold nanospheres: a multi-responsive drug delivery system promoting the killing effect of photothermal therapy against osteosarcoma cells
Nano Research (2023)
-
Can iron chelators ameliorate viral infections?
BioMetals (2023)
-
Enterococcus faecalis thrives in dual-species biofilm models under iron-rich conditions
Archives of Microbiology (2022)
-
Impact of Heavy Metal Toxicity on the Gut Microbiota and Its Relationship with Metabolites and Future Probiotics Strategy: a Review
Biological Trace Element Research (2022)
-
Regulatory effects of transition metals supplementation/deficiency on the gut microbiota
Applied Microbiology and Biotechnology (2021)