Abstract
We report here the discovery of the smallest possible carbon nanotube. This has a diameter of 4 Å, which is the narrowest attainable that can still remain energetically stable, as predicted by theory. These nanotubes are confined inside multiwalled carbon nanotubes and their diameter corresponds to that of a C20 dodecahedron with a single carbon atom at each of its twenty apices. Unlike larger carbon nanotubes, which, depending on their diameter and helicity, can be either metallic or semiconducting, these smallest nanotubes are always metallic.
Similar content being viewed by others
Main
Many of the extraordinary properties of carbon nanotubes1 depend on their diameter. The smallest carbon nanotubes have been associated with the smallest fullerenes2, with nanotube diameters of 7 or 5 Å corresponding to those of C60 and C36 structures, respectively. Carbon nanotubes with such small diameters have been observed experimentally3,4,5.
The carbon nanotubes reported here were seen in cathodic deposits produced by arc-discharge of graphite rods in a hydrogen atmosphere without a metallic catalyst6. Under these conditions, more carbon nanotubes (all multiwalled) are kept open as hydrogen etches away the capping atoms, a unique feature that helps maintain a favourable environment for smaller carbon nanotubes to form inside already-grown multiwalled carbon nanotubes.
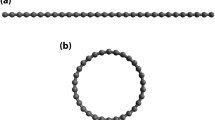
Figure 1 shows a high-resolution transmission electron micrograph of an 18-shell carbon nanotube produced in this way: each dark line represents the side wall of a cylindrical graphene shell in projection and the innermost shell has a diameter of 4 Å. The cylindrical structure of the nanotube is shown by the reduced contrast towards the centre of the nanotube, where there are fewer atoms in the smaller tubes. Multiwalled carbon nanotubes with innermost shells of diameter 5 or 7 Å were also detected in the same material.
Dark lines correspond to the side walls of the single-wall shells. The blurring towards the centre is due to the smaller number of atoms in the smaller tubules. Inset, model for the formation and growth of a [3,3] armchair tubule of 4 Å diameter. Half of the C20 dodecahedron (yellow) serves as a nucleus and further growth into the tubule is realized by adding carbon atoms (blue).
Some of the smallest nanotubes seen were capped, and electron micrographs of the cap of a 4 Å nanotube indicate that the smallest nanotubes have an antichiral [3, 3] 'armchair' structure7 ( Fig. 1, inset). We propose that these 4 Å nanotubes grow out of half of a C20 dodecahedron, in which the C–C bonding angle is 108° — very close to the bonding angle (109.5°) in the sp3 configuration of diamond. Growth into a full nanotube is realized through a step-by-step mechanism on both the outer and inner surfaces8 (Fig. 1, inset). The hydrogen atmosphere facilitates the formation of the halves of the C20 dodecahedron, stabilizing the structure by terminating certain dangling bonds with hydrogen, and keeps the inside of the nanotubes open so that, after the confining outer shells have been formed, carbon species can enter the core to form the innermost shell.
Although these tiny 4 Å carbon nanotubes should be energetically stable9, the severe steric distortion resulting from the planar graphene changes the electronic structure significantly10. Electronic band-structure calculations indicate that all such tubules tend to be metallic, regardless of their helicity.
C20 fullerenes have recently been made using a gas-phase reaction method11. Our finding indicates that C20 fullerenes could also be produced by the arc-discharge method. It remains a challenge to produce single-walled carbon nanotubes of 4 Å diameter experimentally.
References
Iijima, S. Nature 354, 56–58 ( 1991).
Heath, J. R. Nature 393, 730–731 ( 1998).
Ajayan, P. M. & Iijima, S. Nature 358, 23 (1992).
Kataura, H., Achiba, Y., Zhao, X. & Ando, Y. Mat. Res. Soc. Symp. Proc. 593, 113–118 ( 2000).
Sun, L. F. et al. Nature 403, 384 ( 2000).
Zhao, X. et al. Carbon 35, 775–781 (1997).
Qin, L.-C et al. Chem. Phys. Lett. 268, 101– 106 (1997).
Iijima, S., Ajayan, P. M. & Ichihashi, T. Phys. Rev. Lett. 69, 3100– 3103 (1992).
Sawada, S. & Hamada, N. Solid State Commun. 83 , 917–919 (1992).
Blase, X., Benedict, L. X., Shirley, E. L. & Louie, S. G. Phys. Rev. Lett. 72, 1878–1881 (1994).
Prinzbach, H. et al. Nature 407, 60–63 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, LC., Zhao, X., Hirahara, K. et al. The smallest carbon nanotube. Nature 408, 50 (2000). https://doi.org/10.1038/35040699
Issue Date:
DOI: https://doi.org/10.1038/35040699
This article is cited by
-
Band-correlated barrier-hopping conduction in α-NiMoO4 micro-crystals and comparison of its energy storage performance with MWCNT-integrated complex
Journal of Materials Science: Materials in Electronics (2020)
-
Legal and practical challenges in classifying nanomaterials according to regulatory definitions
Nature Nanotechnology (2019)
-
Molecular diffusion replaces capillary pumping in phase-change-driven nanopumps
Microfluidics and Nanofluidics (2019)
-
Current Situation and Prospect of Nanometrology and its Standardization in Indonesia
MAPAN (2018)
-
A Cu-atom-chain current channel with a width of approximately 0.246 nm on (5, 0) single-wall carbon nanotube
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.