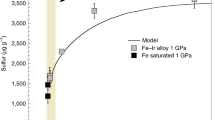
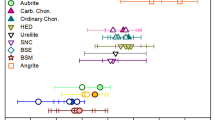
Abstract
The abundances of highly siderophile (iron-loving) elements (HSEs) in the Earth's mantle provide important constraints on models of the Earth's early evolution. It has long been assumed that the relative abundances of HSEs should reflect the composition of chondritic meteorites—which are thought to represent the primordial material from which the Earth was formed. But the non-chondritic abundance ratios recently found in several types of rock derived from the Earth's mantle1,2,3 have been difficult to reconcile with standard models of the Earth's accretion4,5,6,7,8,9, and have been interpreted as having arisen from the addition to the primitive mantle of either non-chondritic extraterrestrial material or differentiated material from the Earth's core. Here we report in situ laser-ablation analyses of sulphides in mantle-derived rocks which show that these sulphides do not have chondritic HSE patterns, but that different generations of sulphide within single samples show extreme variability in the relative abundances of HSEs. Sulphides enclosed in silicate phases have high osmium and iridium abundances but low Pd/Ir ratios, whereas pentlandite-dominated interstitial sulphides show low osmium and iridium abundances and high Pd/Ir ratios. We interpret the silicate-enclosed sulphides as the residues of melting processes and interstitial sulphides as the crystallization products of sulphide-bearing (metasomatic) fluids. We suggest that non-chondritic HSE patterns directly reflect processes occurring in the upper mantle—that is, melting and sulphide addition via metasomatism—and are not evidence for the addition of core material or of ‘exotic’ meteoritic components.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Snow, J. E. & Schmidt, G. Constraints on Earth accretion deduced from noble metals in the oceanic mantle. Nature 391 , 166–169 (1998).
Lorand, J. P., Gros, M. & Pattou, L. Fractionation of platinum-group elements in the upper mantle: a detailed study in Pyrenean orogenic peridotites. J. Petrol. 40, 951–987 ( 1999).
Rehkämper, M. et al. Non-chondritic platinum-group element ratios in oceanic mantle lithosphere: Petrogenetic signature of melt percolation. Earth Planet. Sci. Lett. 172, 65–81 (1999).
Borisov, A., Palme, H. & Spettel, B. Solubility of palladium in silicate melts: implications for core formation in the Earth. Geochim. Cosmochim. Acta 58, 705–716 (1994).
Jagoutz, E. H. et al. The abundance of major, minor and trace elements in the earth's mantle as derived from primitive ultramafic nodules. Proc. Lunar. Planet. Sci. Conf. X, 2031–2050 (1979).
Mitchell, R. H. & Keays, R. R. Abundance and distribution of gold, palladium and iridium in some spinel and garnet lherzolites: implications for the nature and origin of precious metal-rich intergranular components in the upper mantle. Geochim. Cosmochim. Acta 45, 2425–2445 (1981).
Morgan, J. W. Ultramafic xenoliths: clues to earth's late accretionary history. J. Geophys. Res. 91, 12375–12387 (1986).
O'Neill, H. S. C. The origin and the early history of the Earth—A chemical model. Part 2: The Earth. Geochim. Cosmochim. Acta 55, 1159–1172 (1991).
Meisel, T., Walker, R. J. & Morgan, J. W. The osmium isotopic composition of the Earth's primitive upper mantle. Nature 383, 517– 520 (1996).
Pattou, L., Lorand, J. P. & Gros, M. Non-chondritic platinum-group element ratios in the Earth's mantle. Nature 379, 712– 715 (1996).
Shirey, S. B. & Walker, R. J. The Re-Os isotope system in cosmochemistry and high-temperature geochemistry. Annu. Rev. Earth. Planet. Sci. 26, 423–500 ( 1998).
Bulanova, G. P., Griffin, W. L., Ryan, C. G., Shestakova O. Ye. & Barnes, S. J. Trace element in sulfide inclusions from Yakutian diamonds. Contrib. Mineral. Petrol. 124 , 111–125 (1996).
Guo, J., Griffin, W. L. & O'Reilly, S. Y. Geochemistry and origin of sulfide minerals, in mantle xenoliths: Qilin, southeastern China. J. Petrol. 40 , 1125–1149 (1999).
Hart, S. R. & Ravizza, G. E. in Reading the Isotopic Code (eds Basu, A. & Hart, S. R.) 123–134 (American Geophysical Union, Washington DC, 1996).
Lorand, J. P. & Conquéré, F. Contribution a l’étude des sulfures dans les enclaves de lherzolites à spinelle des basaltes alcalins (Massif Central et Languedoc, France). Bull. Minéral. 106, 585–606 ( 1983).
Dromgoole, E. L. & Pasteris, J. D. in Mantle Metasomatism and Alkaline Magmatism (eds Morris, E. & Pasteris, J. D.) 25–46 (Special Paper 215, Geological Society of America, Washington DC, 1987).
Szabó, C. S. & Bodnar, R. J. Chemistry and origin of mantle sulfides in spinel peridotite xenoliths from alkaline basaltic lavas, Nograd-Gomor Volcanic Field, northern Hungary and southern Slovakia. Geochim. Cosmochim. Acta 59, 3917– 3927 (1995).
Mackovicky, M., Mackovicky, E. & Rose-Hansen, J. in Metallogeny of Basic and Ultrabasic Rocks (eds Gallagher, M. J., Ixer, R. A., Neary, C. R. & Prichard, H. M.) 415 –425 (Inst. Min. Metl., London, 1986 ).
Lorand, J. P. Are spinel lherzolite xenoliths representative of the abundance of sulfur in the upper mantle? Geochim. Cosmochim. Acta 54, 1487–1492 (1989).
Ballhaus, C. & Ryan, C. G. Platinum-group elements in the Merensky Reef. 1. PGE in solid solution in base metal sulfides and the down-temperature equilibration history of Merensky ores. Contrib. Mineral. Petrol. 122, 241–251 ( 1995).
Balhaus, C. & Sylvester, P. Noble metal enrichment processes in the Merensky Reef, Bushveld complex. J. Petrol. 41, 546–561 (1999).
Luguet, A. & Lorand, J. P. Minéralogie des sulfures de Fe-Ni-Cu dans les péridotites abyssales de la zone Mark (ride médio-Atlantique, 20-24°N). C.R. Acad. Sci. Paris 329, 637–644 (1999).
Li, C., Barnes, S. J., Mackovicky, E., Rose-Hansen, J. & Mackovicky, M. Partitioning of nickel, copper, iridium, rhenium, platinum and palladium between monosulfide solution and sulphide liquid: Effects of composition and temperature. Geochim. Cosmochim. Acta 60, 1231–1238 (1996).
Peach, C. L., Mathez, E. A., Keays, R. R. & Reeves, S. J. Experimentally-determined sulfide melt-silicate melt partition coefficients for iridium and palladium. Chem. Geol. 117, 361–377 (1994).
Fleet, M. E., Crocket, J. H., Liu, M. & Stone, W. E. Laboratory partitioning of platinum-group elements (PGE) and gold with application to magmatic sulfide–PGE deposits. Lithos 47, 127– 142 (1999).
Burton, K. W., Chiano, P., Birck, J.-L. & Allegre, C. J. Osmium isotope disequilibrium between mantle minerals in a spinel-lherzolite. Earth Planet. Sci. Lett. 172, 311– 322 (1999).
Alard, O., Pearson, N. J., Griffin, W. L., Graham, S. & Jackson, S. E. in Beyond 2000, New Frontiers in Isotope Geoscience, Extended Abstract Volume 1– 5 (Lorne, Australia, 2000).
Kullerud, G., Yund, R. A. & Moh, G. H. Phase relation in the Cu-Fe-Ni, Cu-Ni-S and Fe-Ni-S systems. Econ. Geol. 4, 323– 343 (1969).
McDonough, W. F. & Sun, S. S. The chemical composition of the Earth. Chem. Geol. 120, 223– 253 (1995).
Acknowledgements
We thank F.R. (Joe) Boyd, S. Talnikova, Y. Barashkov and W.J. Powell for providing samples, and N.J. Pearson, C. Lawson and A. Sharma for assistance with the analytical facilities. We thank Y. Lahaye for comments on an earlier version of this Letter, and R. Carlson and M. Rehkämper for comments on the final version. This is a GEMOC National Key Centre publication.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Rights and permissions
About this article
Cite this article
Alard, O., Griffin, W., Lorand, J. et al. Non-chondritic distribution of the highly siderophile elements in mantle sulphides. Nature 407, 891–894 (2000). https://doi.org/10.1038/35038049
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35038049
This article is cited by
-
A geochemical perspective on the genesis of Cenozoic basic volcanism in northeastern Turkey: an overview of metasomatism and heterogeneity of the sub-continental lithospheric mantle in a post-collisional setting
Acta Geochimica (2023)
-
Platinum-group element geochemical constraints on mantle heterogeneity and petrogenesis along the Cameroon Volcanic Line
Arabian Journal of Geosciences (2023)
-
Contrasting platinum-group element geochemistry of post-collisional porphyry Cu ± Au ore-bearing and barren suites in the central and southeastern Urumieh-Dokhtar magmatic arc, Iran
Mineralium Deposita (2023)
-
Crustal magmatic controls on the formation of porphyry copper deposits
Nature Reviews Earth & Environment (2021)
-
Breaking of Henry’s law for sulfide liquid–basaltic melt partitioning of Pt and Pd
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.