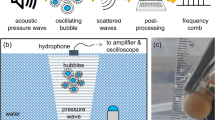
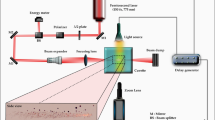
Abstract
Ultrasound can drive a single gas bubble in water into violent oscillation; as the bubble is compressed periodically, extremely short flashes of light (about 100 ps) are generated with clock-like regularity1,2,3,4. This process, known as single-bubble sonoluminescence, gives rise to featureless continuum emission4,5 in water (from 200 to 800 nm, with increasing intensity into the ultraviolet). In contrast, the emission of light from clouds of cavitating bubbles at higher acoustic pressures (multi-bubble sonoluminescence1) is dominated by atomic and molecular excited-state emission6,7,8,9,10,11 at much lower temperatures6. These observations have spurred intense effort to uncover the origin of sonoluminescence and to generalize the conditions necessary for its creation. Here we report a series of polar aprotic liquids that generate very strong single-bubble sonoluminescence, during which emission from molecular excited states is observed. Previously, single-bubble sonoluminescence from liquids other than water has proved extremely elusive12,13. Our results give direct proof of the existence of chemical reactions and the formation of molecular excited states during single-bubble cavitation, and provide a spectroscopic link between single- and multi-bubble sonoluminescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Suslick, K. S. & Crum, L. A. in Encyclopedia of Acoustics (ed. Crocker, M. J.) 271–282 (Wiley-Interscience, New York, 1997).
Gaitan, D. F., Crum, L. A., Church, C. C. & Roy, R. A. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J. Acoust. Soc. Am. 91, 3166– 3183 (1992).
Gompf, B. et al. Resolving sonoluminescence pulse width with time-correlated single photon counting. Phys. Rev. Lett. 79, 1405 –1408 (1997).
Hiller, R. A., Putterman, S. J. & Weninger, K. R. Time-resolved spectra of sonoluminescence. Phys. Rev. Lett. 80, 1090–1093 (1998).
Hiller, R., Putterman, S. J. & Barber, B. P. Spectrum of synchronous picosecond sonoluminescence. Phys. Rev. Lett. 69, 1182– 1184 (1992).
McNamara, W. B. III, Didenko, Y. T. & Suslick, K. S. Sonoluminescence temperatures during multi-bubble cavitation. Nature 401, 772–775 (1999).
Suslick, K. S. & Flint, E. B. Sonoluminescence from non-aqueous liquids. Nature 330, 553– 555 (1987).
Flint, E. B. & Suslick, K. S. Sonoluminescence from nonaqueous liquids: emission from small molecules. J. Am. Chem. Soc. 111, 6987–6992 (1989).
Flint, E. B. & Suslick, K. S. Sonoluminescence from alkali metal salt solutions. J. Phys. Chem. 95, 1484–1488 (1991).
Suslick, K. S., Flint, E. B., Grinstaff, M. W. & Kemper, K. Sonoluminescence from metal carbonyls. J. Phys. Chem. 97, 3098–3099 (1993).
Didenko, Y. T. & Pugach, S. P. Spectra of water sonoluminescence. J. Phys. Chem. 98, 9742 –9749 (1994).
Gaitan, D. F. et al. Spectra of single-bubble sonoluminescence in water and glycerine-water mixtures. Phys. Rev. E 54, 525– 528 (1996).
Weninger, K. et al. Sonoluminescence from single bubbles in nonaqueous liquids: new parameter space for sonochemistry. J. Phys. Chem. 99, 14195–14197 (1995).
Moss, W. C., Clarke, D. B. & Young, D. A. Calculated pulse widths and spectra of a single sonoluminescing bubble. Science 276, 1398– 1401 (1997).
Hilgenfeldt, S., Grossmann, S. & Lohse, D. A simple explanation of light emission in sonoluminescence. Nature 398, 402–405 (1999).
Bernstein, L. S. & Zakin, M. R. Confined electron model for single-bubble sonoluminescence. J. Phys. Chem. 99, 14619–14627 (1995).
Lohse, D. et al. Sonoluminescing air bubbles rectify argon. Phys. Rev. Lett. 78, 1359–1362 ( 1997).
Lohse, D. & Hilgenfeldt, S. Inert gas accumulation in sonoluminescing bubbles. J. Chem. Phys. 107, 6986– 6997 (1997).
Weninger, K. R. et al. Sonoluminescence from an isolated bubble on a solid surface. Phys. Rev. E 56, 6745– 6749 (1997).
Matula, T. J. & Crum, L. A. Evidence for gas exchange in single-bubble sonoluminescence. Phys. Rev. Lett. 80, 865 –868 (1998).
Ketterling, J. A. & Apfel, R. E. Experimental validation of the dissociation hypothesis for single bubble sonoluminescence. Phys. Rev. Lett. 81, 4991– 4994 (1998).
Bernstein, L. S., Zakin, M. S., Flint, E. B. & Suslick, K. S. Cavitation thermometry using molecular and continuum sonoluminescence. J. Phys. Chem. 100, 6612–6619 (1996).
Lide, D. R. (ed.) CRC Handbook of Chemistry and Physics (CRC Press, Boca Raton, 1993–1994).
Gaydon, A. G. The Spectroscopy of Flames 2nd edn (Chapman and Hall, London, 1974).
Hertzberg, G. Molecular Spectra and Molecular Structure: Electronic Spectra and Electronic Structure of Polyatomic Molecules (Van Nostrand, New York, 1966).
Zel’dovich, Y. B. & Raizer, Yu. P. Physics of Shock Waves and High Temperature Hydrodynamic Phenomena (Academic, New York, 1966).
Moran, M. J. & Sweider, D. Measurements of sonoluminescence temporal pulse shape. Phys. Rev. Lett. 80, 4987–4990 (1998).
Acknowledgements
We thank T. J. Matula for the loan of a calibrated needle hydrophone and many discussions, and S. Kulikov for assistance in constructing the imaging system. This work was supported by the US Defense Advanced Research Projects Agency and in part by the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Didenko, Y., McNamara III, W. & Suslick, K. Molecular emission from single-bubble sonoluminescence. Nature 407, 877–879 (2000). https://doi.org/10.1038/35038020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35038020
This article is cited by
-
In vivo ultrasound-induced luminescence molecular imaging
Nature Photonics (2024)
-
Single-Bubble Sonoluminescence as a New Technique for Determination of Metals in Mineral Water
Journal of Applied Spectroscopy (2024)
-
Ultrasound-induced biophysical effects in controlled drug delivery
Science China Life Sciences (2022)
-
Ultrasonic oscillations induced property development of water-bentonite suspension containing sulfonated wood coal
Journal of Petroleum Exploration and Production Technology (2021)
-
A new source of radiation in single-bubble sonoluminescence
Pramana (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.