Abstract
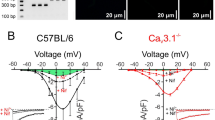
Small arteries exhibit tone, a partially contracted state that is an important determinant of blood pressure. In arterial smooth muscle cells, intracellular calcium paradoxically controls both contraction and relaxation. The mechanisms by which calcium can differentially regulate diverse physiological responses within a single cell remain unresolved. Calcium-dependent relaxation is mediated by local calcium release from the sarcoplasmic reticulum. These ‘calcium sparks’ activate calcium-dependent potassium (BK) channels comprised of α and β1 subunits. Here we show that targeted deletion of the gene for the β1 subunit leads to a decrease in the calcium sensitivity of BK channels, a reduction in functional coupling of calcium sparks to BK channel activation, and increases in arterial tone and blood pressure. The β1 subunit of the BK channel, by tuning the channel's calcium sensitivity, is a key molecular component in translating calcium signals to the central physiological function of vasoregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Knot, H. J., Zimmermann, P. A. & Nelson, M. T. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J. Physiol. (Lond.) 492, 419–430 (1996).
Jaggar, J. H., Porter, V. A., Lederer, W. J. & Nelson, M. T. Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278, C235–C256 ( 2000).
Galvez, A. et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 265, 11083–11090 (1990).
Knot, H. J., Standen, N. B. & Nelson, M. T. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J. Physiol. (Lond.) 508 , 211–221 (1998).
Brayden, J. E. & Nelson, M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256, 532–535 (1992).
Nelson, M. T. & Quayle, J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 268, C799–822 ( 1995).
Sah, P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 19, 150–154 (1996).
Vergara, C., Latorre, R., Marrion, N. V. & Adelman, J. P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8, 321–329 (1998).
Kaczorowski, G. J., Knaus, H. G., Leonard, R. J., McManus, O. B. & Garcia, M. L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr. 28, 255–267 ( 1996).
Latorre, R. in Molecular Workings of Large Conductance (Maxi) Ca2+-Activated K+ Channels 79–102 (Academic, San Diego, 1994).
Brenner, R., Jegla, T. J., Wickenden, A., Liu, Y. & Aldrich, R. W. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275, 6453–6461 (2000).
Behrens, R. et al. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 474, 99– 106 (2000).
Knaus, H. G. et al. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 269, 17274–17278 (1994).
Meera, P., Wallner, M. & Toro, L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl Acad. Sci. USA 97, 5562–5567 (2000).
Wallner, M., Meera, P. & Toro, L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc. Natl Acad. Sci. USA 96, 4137– 4142 (1999).
Xia, X. M., Ding, J. P. & Lingle, C. J. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19, 5255–5264 (1999).
Xia, X. M., Ding, J. P., Zeng, X. H., Duan, K. L. & Lingle, C. J. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J. Neurosci. 20, 4890–4903 (2000).
Riazi, M. A. et al. Identification of a putative regulatory subunit of a calcium-activated potassium channel in the dup(3q) syndrome region and a related sequence on 22q11.2. Genomics 62, 90– 94 (1999).
Weiger, T. M. et al. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J. Neurosci. 20, 3563–3570 ( 2000).
McManus, O. B. et al. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14, 645– 650 (1995).
Knaus, H. G., Eberhart, A., Kaczorowski, G. J. & Garcia, M. L. Covalent attachment of charybdotoxin to the beta-subunit of the high conductance Ca2+-activated K+ channel. Identification of the site of incorporation and implications for channel topology. J. Biol. Chem. 269, 23336–23341 (1994).
Dworetzky, S. I. et al. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J. Neurosci. 16, 4543–4550 (1996).
Cox, D. H. & Aldrich, R. W. Regulation of the large conductance Ca+2-activated K+ channel by its β subunit. J. Gen. Physiol. 116, 411– 432 (2000).
Meera, P., Wallner, M., Jiang, Z. & Toro, L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (K V,Ca beta) of maxi K channels. FEBS Lett. 382 , 84–88 (1996).
Tanaka, Y., Meera, P., Song, M., Knaus, H. G. & Toro, L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J. Physiol. (Lond.) 502, 545– 557 (1997).
Perez, G. J., Bonev, A. D., Patlak, J. B. & Nelson, M. T. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J. Gen. Physiol. 113, 229–238 (1999).
Jiang, Z., Wallner, M., Meera, P. & Toro, L. Human and rodent MaxiK channel beta-subunit genes: cloning and characterization. Genomics 55, 57–67 ( 1999).
Poolos, N. P. & Johnston, D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J. Neurosci. 19, 5205–5212 (1999).
Bayliss, W. M. On the local reactions of the arterial wall to changes in internal pressure. J. Physiol. (Lond.) 28, 220– 231 (1902).
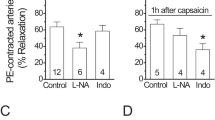
Nelson, M. T. et al. Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637 ( 1995).
Meininger, G. A. & Davis, M. J. Cellular mechanisms involved in the vascular myogenic response. Am. J. Physiol. 263, H647–659 (1992).
Shesely, E. G. et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 93, 13176–13181 (1996).
Desai, K. H. et al. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am. J. Physiol. 272, H1053–1061 (1997).
Wilson, P. W. From hypertension to heart failure: what have we learned? Clin. Cardio. 22 (suppl. 5), V1–10 (1999).
McCobb, D. P. et al. A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am. J. Physiol. 269, H767–777 (1995).
Zhou, X. B., Schlossmann, J., Hofmann, F., Ruth, P. & Korth, M. Regulation of stably expressed and native BK channels from human myometrium by a cGMP- and cAMP-dependent protein kinase. Pflugers Arch. 436, 725– 734 (1998).
Dong, H., Waldron, G. J., Cole, W. C. & Triggle, C. R. Roles of calcium-activated and voltage-gated delayed rectifier potassium channels in endothelium-dependent vasorelaxation of the rabbit middle cerebral artery. Br. J. Pharmacol. 123, 821– 832 (1998).
Khan, S. A., Higdon, N. R. & Meisheri, K. D. Coronary vasorelaxation by nitroglycerin: involvement of plasmalemmal calcium-activated K+ channels and intracellular Ca++ stores. J. Pharmacol. Exp. Ther. 284, 838–846 (1998).
Tybulewicz, V. L., Crawford, C. E., Jackson, P. K., Bronson, R. T. & Mulligan, R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 ( 1991).
Joyner, A. L. in Gene Targeting: A Practical Approach Vol. xviii, 234 (IRL at Oxford Univ. Press, Oxford, New York, 1993 ).
Sambrook, J., Maniatis, T. & Fritsch, E. F. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989 ).
Hogan, B. in Manipulating the Mouse Embryo: A Laboratory Manual Vol. xvii, 497 (Cold Spring Harbor Laboratory Press, Plainview, New York, 1994).
Berkowitz, R. D. et al. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J. Virol. 72, 10108–10117 (1998).
Knot, H. J. & Nelson, M. T. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. (Lond.) 508 , 199–209 (1998).
Nelson, M. T. et al. Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637 ( 1995).
Horn, R. & Marty, A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 92, 145–159 ( 1988).
Simpson, L. O. A mouse model of spontaneous renal hypertension. blood pressure, heart weight, kidney weight and proteinuria relationships in NZB × OUW F1 hybrid female mice. Pathology 12, 347– 357 (1980).
Han, J. et al. Age-related changes in blood pressure in the senescence-accelerated mouse (SAM): aged SAMP1 mice manifest hypertensive vascular disease. Lab. Anim. Sci. 48, 256–263 (1998).
Acknowledgements
We thank B. K. Kobilka of Stanford University for advice; Y. Chen in the Stanford Transgenic Mouse facility for the blastocyst injections; G. Mawe for advice and assistance on isolated artery images; S. Brett Welsh for genotyping and technical assistance; D. Hill-Eubanks for comments on the manuscript and D. P. Regula for paraffin sections of hearts. DHS-1 was provided by Merck Research Laboratories. This work was supported by a grant from the Federation for Anesthesia Education and Research to A.J.P. and by grants from the National Institutes of Health (H.L.B.I. and D.D.K.), National Science Foundation, Totman Medical Trust for Cerebrovascular Research to M.T.N., and an American Heart Association Fellowship to G.P. R.W.A. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brenner, R., Peréz, G., Bonev, A. et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407, 870–876 (2000). https://doi.org/10.1038/35038011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35038011
This article is cited by
-
Progesterone activation of β1-containing BK channels involves two binding sites
Nature Communications (2023)
-
Opposing responses of the rat pulmonary artery and vein to phenylephrine and other agents in vitro
BMC Pulmonary Medicine (2021)
-
Small molecule modulation of the Drosophila Slo channel elucidated by cryo-EM
Nature Communications (2021)
-
Nuclear BK channels regulate CREB phosphorylation in RAW264.7 macrophages
Pharmacological Reports (2021)
-
Inhibition of big-conductance Ca2+-activated K+ channels in cerebral artery (vascular) smooth muscle cells is a major novel mechanism for tacrolimus-induced hypertension
Pflügers Archiv - European Journal of Physiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.