Abstract
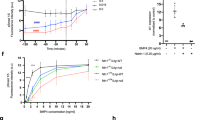
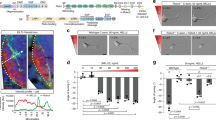
The netrins, a family of laminin-related secreted proteins, are critical in controlling axon elongation and pathfinding1,2,3,4. The DCC (for deleted in colorectal cancer) protein was proposed as a receptor for netrin-1 in the light of many observations including the inhibition of netrin-1-mediated axon outgrowth and attraction in the presence of an anti-DCC antiserum5,6,7, the similitude of nervous system defects in DCC and netrin-1 knockout mice4,8 and the results of receptor swapping experiments9. Previous studies have failed to show a direct interaction of DCC with netrin-1 (ref. 10), suggesting the possibility of an additional receptor or co-receptor. Here we show that DCC interacts with the membrane-associated adenosine A2b receptor, a G-protein-coupled receptor that induces cAMP accumulation on binding adenosine11. We show that A2b is actually a netrin-1 receptor and induces cAMP accumulation on binding netrin-1. Finally, we show that netrin-1-dependent outgrowth of dorsal spinal cord axons directly involves A2b. Together our results indicate that the growth-promoting function of netrin-1 may require a receptor complex containing DCC and A2b.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tessier-Lavigne, M. & Goodman, C. S. The molecular biology of axon guidance. Science 274, 1123 –1133 (1996).
Kennedy, T. E., Serafini, T., de la Torre, J. R. & Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425– 435 (1994).
Colamarino, S. A. & Tessier-Lavigne, M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell 81, 621–629 (1995).
Serafini, T. et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996).
Keino-Masu, K. et al. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell 87, 175– 185 (1996).
Deiner, M. S. et al. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron 19, 575–589 ( 1997).
de la Torre, J. R. et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron 19, 1211 –1224 (1997).
Fazeli, A. et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796– 804 (1997).
Hong, K. et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941 (1999).
Meyerhardt, J. A. et al. Netrin-1: interaction with deleted in colorectal cancer (DCC) and alterations in brain tumors and neuroblastomas. Cell Growth Differ. 10, 35–42 ( 1999).
Feoktistov, I. & Biaggioni, I. Adenosine A2B receptors. Pharmacol. Rev. 49, 381– 402 (1997).
Mehlen, P. et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395, 801– 804 (1998).
Forcet, C. et al. The dependence receptor DCC-mediated mitochondria independent caspase-9 dependent caspase activation. Proc. Natl Acad. Sci. USA , submitted
Ralevic, V. & Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413– 492 (1998).
Feoktistov, I. & Biaggioni, I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J. Clin. Invest. 96, 1979–1986 (1995).
Yakel, J. L., Warren, R. A., Reppert, S. M. & North, R. A. Functional expression of adenosine A2b receptor in Xenopus oocytes. Mol. Pharmacol. 43, 277– 280 (1993).
Squires, R. F. et al. Honokiol and magnolol increase the number of [3H]muscimol binding sites three-fold in rat forebrain membranes in vitro using a filtration assay, by allosterically increasing the affinities of low-affinity sites. Neurochem. Res. 24, 1593– 1602 (1999).
Boileau, A. J., Evers, A. R., Davis, A. F. & Czajkowski, C. Mapping the agonist binding site of the GABAA receptor: evidence for a beta-strand. J. Neurosci. 19, 4847– 4854 (1999).
Ming, G. L. et al. cAMP-dependent growth cone guidance by netrin-1. Neuron 19, 1225–1235 ( 1997).
Hopker, V. H., Shewan, D., Tessier-Lavigne, M., Poo, M. & Holt, C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401, 69–73 (1999).
Feoktistov, I. & Biaggioni, I. Characterization of adenosine receptors in human erythroleukemia cells and platelets: further evidence for heterogeneity of adenosine A2 receptor subtypes. Mol. Pharmacol. 43, 909–914 (1993).
Fredholm, B. B. & Persson, C. G. Xanthine derivatives as adenosine receptor antagonists. Eur. J. Pharmacol. 81, 673–676 (1982).
Song, H. et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515–1518 (1998).
Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994).
Song, H. & Poo, M. Signal transduction underlying growth cone guidance by diffusible factor. Curr. Opin. Neurobiol. 9, 355–363 (1999).
Bennett, K. L. et al. Deleted in colorectal carcinoma (DCC) binds heparin via its fifth fibronectin type III domain. J. Biol. Chem. 272 , 26940–26946 (1997).
Daly, J. W., Butts-Lamb, P. & Padgett, W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell. Mol. Neurobiol. 3, 69–80 (1983)
Linden, J., Thai, T., Figler, H., Jin, X. & Robeva, A. S. Characterization of human A(2B) adenosine receptors: radioligand binding, western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol. Pharmacol. 56, 705–713 (1999).
de Castro, F., Hu, L., Drabkin, H., Sotelo, C. & Chedotal, A. Chemoattraction and chemorepulsion of olfactory bulb axons by different secreted semaphorins. J. Neurosci. 19, 4428–4436 (1999).
Acknowledgements
We thank L. Granger for technical assistance, P. Schofield for the human A2b expressing construct, and M. Tessier-Lavigne for the purified netrin-1. This work was supported by the CNRS, the Ligue Contre le Cancer, the FRM, the ARC (to P.M. and to A.C.), the ‘emmergence’ program (to P.M.), and APEX INSERM (to A.C.). K.T.N.-B.-C is a recipient of a postdoctoral fellowship from IRP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corset, V., Nguyen-Ba-Charvet, K., Forcet, C. et al. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature 407, 747–750 (2000). https://doi.org/10.1038/35037600
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35037600
This article is cited by
-
Neogenin suppresses tumor progression and metastasis via inhibiting Merlin/YAP signaling
Cell Death Discovery (2023)
-
Netrin-4: Focus on Its Role in Axon Guidance, Tissue Stability, Angiogenesis and Tumors
Cellular and Molecular Neurobiology (2023)
-
Adenosine as a Key Mediator of Neuronal Survival in Cerebral Ischemic Injury
Neurochemical Research (2022)
-
Roles and Mechanisms of Axon-Guidance Molecules in Alzheimer’s Disease
Molecular Neurobiology (2021)
-
Serotonin 5-HT4 receptor boosts functional maturation of dendritic spines via RhoA-dependent control of F-actin
Communications Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.