Abstract
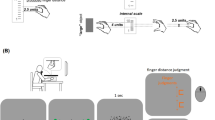
People adapt with remarkable flexibility to reversal of the visual field caused by prism spectacles1,2. With sufficient time, this adaptation restores visually guided behaviour and perceptual harmony between the visible and tactile worlds1,2,3. Although it has been suggested that seeing one's own body is crucial for adaptation1,2, the underlying mechanisms are unclear. Here we show that a new representation of visuomotor mapping with respect to the hands emerges in a month during adaptation to reversed vision. The subjects become bi-perceptual3,4,5, or able to use both new and old representations. In a visual task designed to assess the new hand representation, subjects identified visually presented hands as left or right by matching the picture to the representation of their own hands. Functional magnetic resonance imaging showed brain activity in the left posterior frontal cortex (Broca's area) that was unique to the new hand representations of both hands, together with activation in the intraparietal sulcus and prefrontal cortex. The emergence of the new hand representation coincided with the adaptation of perceived location of visible objects in space. These results suggest that the hand representation operates as a visuomotor transformation device that provides an arm-centred frame of reference6 for space perception.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kohler, I. The formation and transformation of the perceptual world. Psychol. Issues 3 (monogr. 12) 1–173 (1964).
Stratton, G. M. Vision without inversion of the retinal image. Psychol. Rev. 4, 341–360; 463–481 ( 1897).
Sugita, Y. Global plasticity in adult visual cortex following reversal of visual input. Nature 380, 523–526 (1996).
Miyauchi, S. et al. Adaptation to reversing prisms activates the ipsilateral visual cortex in humans: an fMRI study. Invest. Ophthalmol. Vis. Sci. 40, S820 (1999).
Held, R. Shifts in binaural localization after prolonged exposure to atypical combination of stimuli. Am. J. Psychol. 68, 526– 548 (1955).
Graziano, M. S. A., Yap, G. S. & Gross, C. G. Coding of visual space by premotor neurons. Science 266, 1054–1057 ( 1994).
Sekiyama, K. Kinesthetic aspects of mental representations in the identification of left and right hands. Percept. Psychophys. 32, 89–95 (1982).
Sekiyama, K. Mental and physical movements of hands: Kinesthetic information preserved in representational systems. Jpn. Psychol. Res. 25, 95–102 (1983).
Bonda, E., Petrides, M., Frey, S. & Evans, A. Neural correlates of mental transformations of the body-in-space. Proc. Natl Acad. Sci. USA 92, 11180–11184 (1995).
Parsons, L. M. et al. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature 375, 54– 58 (1995).
Roland, P. E. Brain Activation (Wiley-Liss, New York, 1993).
Kosslyn, S. M., DiGirolamo, G. J., Thompson, W. L. & Alpert, N. M. Mental rotation of objects versus hands: neural mechanisms revealed by positron emission tomography. Psychophysiology 35, 151–161 (1998).
Alivisatos, B. & Petrides, M. Functional activation of the human brain during mental rotation. Neuropsychologia 35, 111–1118 (1997).
Jeannerod, M., Arbib, M. A., Rizzolatti, G. & Sakata, H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 18, 314–320 (1995).
Selemon, L. D. & Goldman-Rakic, P. S. Common cortical subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. J. Neurosci. 8, 4049–4068 (1988).
Murray, E. A., Bussey, T. J. & Wise, S. P. Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Exp. Brain Res. 1, 114 –129 (2000).
White, I. M. & Wise, S. P. Rule-dependent neuronal activity in the prefrontal cortex. Exp. Brain Res. 126, 315–335 (1999).
Cohen, J. D. et al. Temporal dynamics of brain activation during a working memory task. Nature 386, 604–608 (1997).
Courtney, S. M., Ungerleider, L. G., Keil, K. & Haxby, J. V. Transient and sustained activity in a distributed neural system for human working memory. Nature 386, 608– 611 (1997).
Perenin, M. T. & Vighetto, A. Optic ataxia: A specific disruption in visuomotor mechanisms. Brain 111, 643–674 (1988).
Clower, D. M. et al. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature 383, 618 –621 (1996).
Snyder, L. H., Batista, A. P. & Andersen, R. A. Coding of intention in the posterior parietal cortex. Nature 386, 167–170 (1997).
Rushworth, M. F. S., Nixon, P. D. & Passingham, R. E. Parietal cortex and movement: I. Movement selection and reaching. Exp. Brain Res. 117, 292– 310 (1997).
Taira, M., Mine, S., Georgopoulos, A. P., Murata, A. & Sakata, H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp. Brain Res. 83, 29–36 ( 1990).
Iriki, A., Tanaka, M. & Iwamura, Y. Coding of modified body schema during tool use by macaque postcentral neurons. NeuroReport 7, 2325 –2330 (1996).
Krams, M., Rushworth, M. F. S., Deiber, M. P., Frackowiak, R. S. J. & Passingham, R. E. The preparation, execution and suppression of copied movements in the human brain. Exp. Brain Res. 120, 386–398 (1998).
Grafton, S. T., Arbib, M. A., Fadiga, L. & Rizzolatti, G. Localization of grasp representations in humans by positron emission tomography: 2. Observation compared with imagination. Exp. Brain Res. 112, 103–111 (1996).
Rizzolatti, G., Fadiga, L., Gallese, V. & Fogassi, L. Premotor cortex and the recognition of motor actions. Cogn. Brain Res. 3, 131–141 (1996).
Kurata, K. & Hoshi, E. Reacquisition deficits in prism adaptation after muscimol microinjection into the ventral premotor cortex of monkeys. J. Neurophysiol. 81, 1927– 1938 (1999).
Acknowledgements
We thank M. Kato, T. Hayakawa, T. Murata, H. Tanabe and M. Nakatsuka for technical assistance and discussions.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Sekiyama, K., Miyauchi, S., Imaruoka, T. et al. Body image as a visuomotor transformation device revealed in adaptation to reversed vision. Nature 407, 374–377 (2000). https://doi.org/10.1038/35030096
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35030096
This article is cited by
-
Adaptive changes in automatic motor responses based on acquired visuomotor correspondence
Experimental Brain Research (2019)
-
A wearable system for adaptation to left–right reversed audition tested in combination with magnetoencephalography
Biomedical Engineering Letters (2017)
-
Adapting to inversion of the visual field: a new twist on an old problem
Experimental Brain Research (2013)
-
Relationship Between Brain Abnormalities and Cognitive Profile in Williams Syndrome
Behavior Genetics (2011)
-
Somatosensory conflicts in complex regional pain syndrome type 1 and fibromyalgia syndrome
Current Rheumatology Reports (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.