Puckering in a ticklish plant is controlled by dephosphorylation of its actin.
Abstract
The phosphorylation of the amino acid tyrosine in animal proteins acts as an on–off switch in numerous pathways that regulate growth, differentiation and oncogenesis1,2. In the unicellular slime mould Dictyostelium , tyrosine-phosphorylation of actin controls cell-shape changes and spore activity3,4, but the significance of protein-tyrosine phosphorylation in plants is unknown5. Here we show that actin in the contact-sensitive plant Mimosa pudica L. is heavily tyrosine-phosphorylated and that changes in the extent of phosphorylation correlate with the degree of bending of the plant's petioles. We propose that tyrosine-phosphorylation of actin controls movements in plants.
Similar content being viewed by others
Main
M. pudica closes its leaves and points its petiole downwards when touched (Fig. 1a,b). Petiole bending is caused by a rapid shrinking of the lower side of motor organs called the main pulvinus6,7,8 (Fig. 1c,d). Cytochalasin B and phalloidin, which both have inhibitory effects on the actin cytoskeleton, inhibit the bending of the petiole, suggesting that actin is involved in bending9. We found that actin filaments in the motor cells at the lower side of the pulvinus, but not at the upper side, become more peripheral after bending (data not shown), although it is not known what molecular mechanism regulates these changes.
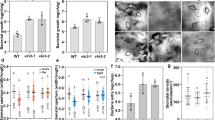
We carried out two-dimensional polyacrylamide gel electrophoresis on total proteins from the whole pulvinus before and after bending, and immunoblotted them with the anti-actin antibody N350 and reprobed with the anti-phosphotyrosine antibody PY20. Densitometry indicated that, before movement, about 80% of actin molecules in the pulvinus were tyrosine-phosphorylated ( Fig. 2a,b). After movement, the total amount of actin did not change, but immunoblots with N350 and PY20 on one-dimensional polyacrylamide gel electrophoresis showed that the amount of tyrosine phosphorylation in actin had dropped (data not shown).
a–f, Total proteins from the whole pulvinus before (a–c ) and after (d–f) petiole bending were immunoblotted with the anti-actin antibody N350 (a,d) or anti-phosphotyrosine antibody PY20 (b,e), or were silver-stained (c,f) after two-dimensional polyacrylamide gel electrophoresis (IEF, isoelectric focusing; SDS, denaturing polyacrylamide gel). Four actin spots were detected (numbered): spots 1–3 are tyrosine-phosphorylated, spot 4 is not. g–j, Phosphorylation of actin from petioles before the plant is touched (g and j, lane 1), and after touching (h,i) in the absence (h and j, lane 2) or presence (i and j, lane 3) of phenylarsine oxide (PAO), a protein-tyrosine phosphatase inhibitor. Dotted lines indicate the original position of the petiole; arrowheads, actin bands.
Consistent with this decrease in tyrosine phosphorylation, we found that phosphorylated actin in spot 1, the most acidic isoform, disappeared (Fig. 2d–f) — probably because it had been dephosphorylated and shifted to a more basic isoform as a result of bending. In contrast, actin molecules in the stem, which does not move, were heavily tyrosine-phosphorylated, and the level of phosphorylation did not change during movement (data not shown). Changes in the levels of tyrosine phoshorylation of actin in the pulvinus therefore coincide with petiole bending.
In Dictyostelium spores, a decrease in actin phosphorylation is a prerequisite for recovering the dynamic actin cytoskeleton involved in spore germination. A specific inhibitor of protein-tyrosine phosphatases, phenylarsine oxide, inhibits the dephosphorylation of actin and the restoration of cell motility during germination4. We investigated the effects of phenylarsine oxide on M. pudica. When touched, the average bending angle of petioles treated with phenylarsine oxide (Fig. 2g–i ) was smaller (37.3±20.24 degrees; n = 25 ) than that of controls (99.0±4.24 degrees). There was no decrease in the amount of actin phosphorylation following stimulation for the pulvinus exposed to phenylarsine oxide (Fig. 2j).
The bending of M. pudica is therefore correlated with reduced actin phosphorylation in the pulvinus. We propose that actin phosphorylation is essential for petiole bending resulting from actin cytoskeletal alterations. Actin may also be phosphorylated during the opening and closing of guard cells, as actin is dynamically arranged in these cells10.
References
Sun, H. & Tonks, N. K. Trends Biochem. Sci. 19, 480–485 (1994).
Boyle, W. J. Curr. Opin. Oncol. 4, 156–162 (1992).
Howard, P. K., Sefton, B. M. & Firtel, R. A. Science 259, 241– 244 (1993).
Kishi, Y., Clements, C., Mahadeo, D. C., Cotter, D. A. & Sameshima, M. J. Cell Sci. 111, 2923–2932 (1998).
Barizza, E., Lo Schiavo, F., Terzi, M. & Filippini, F. FEBS Lett. 447, 191–194 (1999).
Datta, M. & Dutt, A. K. Nature 179, 253–254 (1957).
Toriyama, H. & Sato, S. Cytologia 36, 359–375 (1971).
Tamiya, T. et al. J. Biochem. 104, 5–8 (1988).
Fleurat-Lessard, P., Roblin, G., Bonmort, J. & Besse, C. J. Exp. Bot. 39, 209–221 (1988).
Eun, S. O. & Lee, Y. Plant Physiol. 115, 1491–1498 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kameyama, K., Kishi, Y., Yoshimura, M. et al. Tyrosine phosphorylation in plant bending. Nature 407, 37 (2000). https://doi.org/10.1038/35024149
Issue Date:
DOI: https://doi.org/10.1038/35024149
This article is cited by
-
Bioinspired strategies for biomimetic actuators from ultrafast to ultraslow
Nano Research (2024)
-
Pulvinus and petiole comparative anatomy in a species of Mimosa with slow nyctinastic leaf movement
Brazilian Journal of Botany (2023)
-
Low molecular weight protein phosphatase APH mediates tyrosine dephosphorylation and ABA response in Arabidopsis.
Stress Biology (2022)
-
Rapid movements in plants
Journal of Plant Research (2021)
-
Metabolic changes in transgenic maize mature seeds over-expressing the Aspergillus niger phyA2
Plant Cell Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.