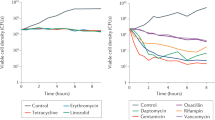
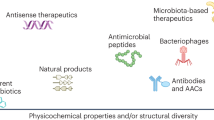
Abstract
Antibiotics — compounds that are literally ‘against life’ — are typically antibacterial drugs, interfering with some structure or process that is essential to bacterial growth or survival without harm to the eukaryotic host harbouring the infecting bacteria. We live in an era when antibiotic resistance has spread at an alarming rate1,2,3,4 and when dire predictions concerning the lack of effective antibacterial drugs occur with increasing frequency. In this context it is apposite to ask a few simple questions about these life-saving molecules. What are antibiotics? Where do they come from? How do they work? Why do they stop being effective? How do we find new antibiotics? And can we slow down the development of antibiotic-resistant superbugs?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neu, H.C. The crisis in antibiotic resistance. Science 257, 1064–1073 (1992).
Gold, H. S. & Moellering, R.C. Antimicrobial-drug resistance . N. Engl. J. Med. 335, 144– 1453 (1996).
Levy, S.B. The challenge of antibiotic resistance. Sci. Am. 275 , 46–53 (1998).
Levy, S.B. Multidrug resistance—a sign of the times. N. Engl. J. Med. 338, 1376–1378 ( 1998).
Spratt, B. G. & Cromie, K. D. Rev. Infect. Dis. 10, 699 (1988).
Williams, D.H. The glycopeptide story—how to kill the deadly “superbugs” . Nat. Prod. Rep. 13, 469– 477 (1996).
Brisson-Noel, A., Trieu-Cuot, P. & Courvalin, P. Mechanism of action of spiramycin and other macrolides . J. Antimicrob. Chemother. 22(Suppl. B), 13–23 (1988).
Chopra, I. in The Tetracyclines, Handbook of Experimental Pharmacology Vol 78 (eds Hlavaka, J. J. & Boothe, J. H.) 317– 392 (Springer, Berlin, 1985).
Fourmy, D., Recht, M. I., Blanchard, S. C. & Puglisi, J.D. Structure of the A site of the Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science 274, 1371–1376 (1996).
Kloss, P., Xiong, L., Shinabarger, D. L. & Mankin, A.S. Resistance of mutations in 23S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center . J. Mol. Biol. 294, 93– 101 (1999).
Shen, L.L. in Quinolone Antibacterial Agents, 2nd edn (eds Hooper, D. C. & Wolfson, J. S.) 77 (American Society for Microbiology, Washington DC, 1993).
Maxwell, A. DNA gyrase as a drug target. Trends Microbiol. 5, 102–109 (1997).
Ferrero, L., Cameron, B. & Crouzet, J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutant of Staphylococcus aureus . Antimicrob. Agents Chemother. 39, 1554–1558 (1995).
Davies, J. Bacteria on the rampage. Nature 383, 219 –220 (1996).
Murray, E. Vancomycin resistant enterococci. Am. J. Med. 102, 284–293 (1997).
Arthur, M. & Courvalin, P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 37, 1563–1571 (1993).
Walsh, C, Fisher, S. L., Park, I. S., Prahalad, M. & Wu, Z. Bacterial resistance to vancomycin: five genes and one missing hydrogen bond tell the story. Chem. Biol. 3, 21–28 (1996 ).
Davies, J. Inactivation of antibiotics and the dissemination of resistance genes. Science 264, 375–382 ( 1994).
Schentag, J.J. et al. Genesis of methicillin resistant Staphylococcus aureus (MRSA). Clin. Infect. Dis. 26, 1204 –1212 (1998).
Jacoby, G. A. & Archer, G.L. New mechanisms of resistance to antibacterial agents. N. Engl. J. Med. 324, 601–612 (1991).
Levy, S.B. Active efflux mechanisms for antimicrobial resistance. Antimicrob. Agents Chemother. 36, 695–703 (1992).
Paulsen, I. T., Brown, M. H. & Skurray, R.A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60, 575–608 (1996).
Ross, J. et al. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super gene family. Mol. Microbiol. 4, 1207–1214 (1990).
Philippon, A., Labia, R. & Jacoby, G. Extended spectrum beta-lactamases. Antimicrob. Agents Chemother. 28, 302–307 (1985).
Shaw, K. J., Rather, P. N., Hare, S. R. & Miller, G. H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57, 138–163 (1993).
Hon, W. C. et al. Structure of an enzyme required for aminoglycoside antibiotic resistance reveals homology to eukaryotic protein kinases. Cell 89, 887–895 ( 1997).
Bussiere, D. et al. Crystal structure of ErmC′, an rRNA methyltransferase which mediates antibiotic resistance in bacteria. Biochemistry 37, 7103–7112 ( 1998).
Bugg, T. D. H. et al. Molecular basis for vancomycin resistance in Enterococcus faecium, BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30, 10408–10415 (1991).
Song, M. D., Wachi, M., Doi, M., Ishino, F. & Matsuhashi, M. Evolution of an inducible penicillin target protein in MRSA by gene fusion. FEBS Lett. 221, 167 (1987).
Spratt, B. G. Resistance to antibiotics mediated by target alterations. Science 264, 388–393 ( 1994).
Knowles, J. R. Penicillin resistance: the chemistry of beta-lactamase inhibition. Acc. Chem. Res. 18, 97–105 (1985).
The choice of antibacterial drugs. Med. Lett. 41, 95–104 ( 1999).
Nelson, M. L., Park, B. H. & Levy, S. B. Molecular requirement for the inhibition of the tetracycline antiport protein and the effect of potent inhibitors on the growth of tetracycline-resistant bacteria. J. Med. Chem. 37, 1355– 1361 (1994).
Chu, D. W., Plattner, J. J. & Katz, L. New directions in antibacterial research. J. Med. Chem. 39, 3853–3874 (1996).
Agouridas, C., Benedetti, Y., Denis, A., Le Martert, O. & Chantot, J. F. Ketolides: a new distinct class of macrolide antibacterials, synthesis and structural characteristics of RU 004. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy Abstr. F158 (American Society for Microbiology, 1995).
Biavasco, F. et al. In vitro antibacterial activity of LY333328, a new semisynthetic glycopeptide. Antimicrob. Agents Chemother. 41, 2165–2172 (1997).
Williams, D. H., Maguire, A. J., Tsuzuki, W. & Westwell, M. S. An analysis of the origins of cooperative binding energy of dimerization. Science 280, 711–713 ( 1998).
Ge, M. et al. Vancomycin derivatives that inhibit peptidoglycan biosynthesis without binding D-Ala-D-Ala. Science 284, 507–511 (1999).
Walsh, C. Deconstructing vancomycin. Science 284, 442–443 (1999).
Barry, A. L. & Fuchs, P. C. In vitro activities of a streptogramin (RP59500), three macrolides, and an azolide against four respiratory tract pathogens. Agents Chemother. 39, 238– 240 (1995).
Linden, P. K., Pasculle, A. W., McDevitt, D. & Kramer, D. J. Effect of quinupristin/dalfopristin (synercid) treatment of infections caused by vancomycin-resistant Enterococcus faecium (VREF). J. Antimicrob. Chemother. 39(Suppl. A), 145 –151 (1997).
Chien, J. W., Kucia, M. L. & Salata, R. A. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin. Infect. Dis. 30, 146–151 (2000).
Kurz, M. & Guba, W. 3D structure of ramoplanin: a potent inhibitor of bacterial cell wall biosynthesis. Biochemistry 35, 12570–12575 (1996).
Chapra, I., Hodgson, J., Metcalf, B. & Poste, G. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob. Agents Chemother. 41, 497– 503 (1997).
Chan, M. K. et al. Crystal structure of E. coli peptide deformylase. Biochemistry 36, 13904–13909 (1997).
Chen, D. Z. et al. Actinonin, a naturally occurring antibacterial agent is a potent peptide deformylase inhibitor. Biochemistry 39, 1256–1262 (2000).
Barret, J. F. & Isaacson, R. E. Bacterial virulence as a potential target for therapeutic intervention. Annu. Rep. Med. Chem. 30, 111–118 (1995).
Stephens, C. & Shapiro, L. Bacterial protein secretion—a target for new antibiotics? Chem. Biol. 4, 637–641 (1997).
Barret, J. F. & Hoch, J. A. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42, 1529–1536 (1998).
Kleerebezem, M., Quadri, L. E. N., Kuipers, O. P. & de Vos, W. M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol. Microbiol. 24, 895–904 (1997).
Reimmann, C. et al. The global activator gacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24, 309– 319 (1997).
Sofia, M. J. et al. Discovery of novel disaccharide antibacterial agents using a combinatorial library approach. J. Med. Chem. 42, 8194–8198 (1999).
Tan, D. S., Foley, M. A., Shair, M. D. & Schreiber, S. L. Stereoselective synthesis of over two million compounds having structural features both reminiscent of natural products and compatible with miniaturized cell-based assays. J. Am. Chem. Soc. 120, 8565–8566 (1998).
Leadlay, P. F. Combinatorial approaches to polyketide biosynthesis. Curr. Opin. Chem. Biol. 1, 162–168 ( 1997).
Katz, L. Manipulation of modular polyketide synthases. Chem. Rev. 97, 2557–2575 (1997).
Khosla, C. Harnessing the biosynthetic potential of modular polyketide synthases. Chem. Rev. 97, 2577–2590 (1997).
Cane, D. E., Walsh, C. T. & Khosla, C. Harnessing the biosynthetic code: combinations, permutations, and mutations. Science 282, 63– 68 (1998).
McDaniel, R. et al. Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel "unnatural" natural products. Proc. Natl Acad. Sci. USA 96, 1846– 1851 (1999).
Zhao, L. et al. Engineering a methymycin/pikromycin-calicheamicin hybrid: construction of two new macrolides carrying a designed sugar moiety. J. Am. Chem. Soc. 121, 9881–9882 (1999).
Schlaes, D.M. et al. Guidelines for the prevention of antimicrobial resistance in hospitals. Clin. Infect. Dis. 25, 583 –599 (1997).
Waldvogel, F. A. New resistance in Staphylococcus aureus. N. Engl. J. Med. 340, 556–557 ( 1999).
Drugs for HIV infection. Med. Lett. 42, 1–6 (2000 ).
Witte, W. Medical consequences of antibiotic use in agriculture. Science 279, 996–997 ( 1998).
Chow, J. W., Donahedian, S. M. & Zervos, M. J. Emergence of increased resistance to quinupristin/dalfopristin during therapy for Enterococcus faecium bacteremia. Clin. Infect. Dis. 24, 90–91 ( 1997).
Acknowledgements
Experimental work cited in this review is supported by the National Institutes of Health. I thank R. Chen and I. Lessard for preparation of the artwork.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000). https://doi.org/10.1038/35021219
Published:
Issue Date:
DOI: https://doi.org/10.1038/35021219
This article is cited by
-
Synthesis of vancomycin fluorescent probes that retain antimicrobial activity, identify Gram-positive bacteria, and detect Gram-negative outer membrane damage
Communications Biology (2023)
-
Antibiotic Resistance Microbes’ (ARM) Mechanisms and Management: A Phytomedicinal Approach
Proceedings of the National Academy of Sciences, India Section B: Biological Sciences (2023)
-
Multi-drug resistant ESKAPE pathogens and the uses of plants as their antimicrobial agents
Archives of Microbiology (2023)
-
Uropathogenic Escherichia coli endeavors: an insight into the characteristic features, resistance mechanism, and treatment choice
Archives of Microbiology (2023)
-
Phytoremediation as a Tool to Remove Drivers of Antimicrobial Resistance in the Aquatic Environment
Reviews of Environmental Contamination and Toxicology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.