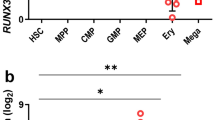
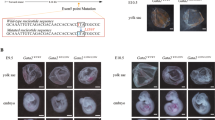
Abstract
GATA-1 is a tissue-specific transcription factor that is essential for the production of red blood cells1,2. Here we show that overexpression of GATA-1 in erythroid cells inhibits their differentiation, leading to a lethal anaemia. Using chromosome-X-inactivation of a GATA-1 transgene and chimaeric animals, we show that this defect is intrinsic to erythroid cells, but nevertheless cell nonautonomous. Usually, cell nonautonomy is thought to reflect aberrant gene function in cells other than those that exhibit the phenotype3. On the basis of our data, we propose an alternative mechanism in which a signal originating from wild-type erythroid cells restores normal differentiation to cells overexpressing GATA-1 in vivo. The existence of such a signalling mechanism indicates that previous interpretations of cell-nonautonomous defects may be erroneous in some cases and may in fact assign gene function to incorrect cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pevny, L. et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349, 257–260 (1991).
Weiss, M. J., Keller, G. & Orkin, S. H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1- embryonic stem cells. Genes Dev. 8, 1184–1197 (1994).
Rossant, J. & Spence, A. Chimera and mosaics in mouse mutant analysis. Trends Genet. 14, 358– 363 (1998).
Weiss, M. J. & Orkin, S. H. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl Acad. Sci. USA 92, 9623– 9627 (1995).
Takahashi, S. et al. Role of GATA-1 in proliferation and differentiation of definitive erythroid and megakaryocytic cells in vivo. Blood 92 , 434–442 (1998).
Whyatt, D. J. et al. The level of the tissue-specific factor GATA-1 affects the cell-cycle machinery. Genes Funct. 1, 11 –24 (1997).
Kuroda, M. I. & Meller, V. H. Transient Xist-ence. Cell 91, 9–11 ( 1997).
Fujiwara, Y., Browne, C. P., Cunniff, K., Goff, S. C. & Orkin, S. H. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA 93, 12355– 12358 (1996).
Tsai, F. -Y., Browne, C. P. & Orkin, S. H. Knock-in mutation of transcription factor GATA-3 into the GATA-1 locus: partial rescue of GATA-1 loss of function in erythroid cells. Dev. Biol. 196, 218– 227 (1998).
Panning, B., Dausman, J. & Jaenisch, R. X chromosome inactivation is mediated by Xist RNA stabilization. Cell 90, 907– 916 (1997).
Sheardown, S. A. et al. Stabilization of Xist RNA mediates initiation of X chromosome inactivation. Cell 91, 99– 107 (1997).
Hendriks, R. W. et al. Inactivation of Btk by insertion by insertion of lacZ reveals defects in B cell development only past the pre-B cell stage. EMBO J. 15, 4862–4872 (1996).
Cormack, D. Time-lapse characterization of erythrocytic colony-forming cells in plasma cultures. Exp. Hematol. 4, 319– 327 (1976).
Sawada, K., Krantz, S. B., Dessypris, E N., Koury, S. T. & Sawyer, S. T. Human colony-forming units-erythroid do not require acessory cells but do require direct interaction with insulin-like growth factor 1 and/or insulin for erythroid development. J. Clin. Invest. 83, 1701–1709 ( 1989).
Apfeld, J. & Kenyon, C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 95, 199–210 ( 1998).
Dubart, A., Romeo, P. H., Vainchenker, W. & Dumenil, D. Constitutive expression of GATA-1 interferes with the cell-cycle regulation. Blood 87, 3711–3721 (1996).
Briegel, K. et al. Regulation and function of transcription factor GATA-1 during red blood cell differentiation. Development 122, 3839–3850 (1996).
Bessis, M., Lessin, L. S. & Beutler, E. in Hematology (eds Williams, W. J., Beutler, E., Erslev, A. J. & Lichtman, M. A.) 257–279 (McGraw-Hill, New York, 1983).
Bernard, J. The erythroblastic island: past and future. Blood Cells 17, 5–14 (1991).
De Maria, R. et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 401, 489– 493 (1999).
Williams, B. O. et al. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13, 4251–4259 (1994).
Maandag, E. C. et al. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13, 4260–4268 (1994).
Hu, N., Gulley, M. L., Kung, J. T. & Lee, E. Y.-H. Retinoblastoma gene deficiency has mitogenic but not tumorigenic effects on erythropoiesis. Cancer Res. 57, 4123– 4129 (1997).
Elefanty, A. G., Antoniou, M., Custodio, N., Carmo-Fonseca, M. & Grosveld, F. G. GATA transcription factors associate with a novel class of nuclear bodies in erythroblasts and megakaryocytes. EMBO J. 15, 319–333 (1996).
Nagamine, C. M., Chan, K. M., Kozak, C. A. & Lau, Y. F. Chromosome mapping and expression of a putative testis-determining gene in the mouse. Science 243, 80– 83 (1989).
Andrews, N. C. & Faller, D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19, 2499 (1991).
Pandolfi, P. P. et al. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nature Genet. 11, 40–44 ( 1995).
Mulder, M. P. et al. Positional mapping of loci in the DiGeorge critical region at chromosome 22q11 using a new marker (D22S183). Human Genet. 96, 133–141 ( 1995).
Wijgerde, M., Grosveld, F. & Fraser, P. Transcription complex stability and chromatin dynamics in vivo. Nature 377, 209– 213 (1995).
Wong, P. M. C., Chung, S. W., Chui, D. H. K. & Eaves, C. J. Properties of the earliest clonogenic hemopoietic procursors to appear in the developing murine yolk sac. Proc. Natl Acad. Sci. USA 83, 3851–3854 (1986).
Acknowledgements
We thank R. Bernards for his critical evaluation, discussions and materials contributing to this work. We also thank M. von Lindern, T. Verkerk, V. Lui, R. Delwel, S. Verbakel, G. Zafarana, N. Gillemans, D. Meijer, T. Stijnen, E. Dzierzak, E. Noteboom, R. Kerkhoven, J.-H. Dannenberg, H. te Riele and members of R. Bernards’ laboratory for advice, assistance and materials at various stages of the project. D.W. is supported by a long-term EMBO Fellowship. F.L. is supported by NWO (Netherlands). R.H. is supported by KNAW (Netherlands). NWO (Netherlands) and the EC supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whyatt, D., Lindeboom, F., Karis, A. et al. An intrinsic but cell-nonautonomous defect in GATA-1-overexpressing mouse erythroid cells. Nature 406, 519–524 (2000). https://doi.org/10.1038/35020086
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35020086
This article is cited by
-
MS4A3-HSP27 target pathway reveals potential for haematopoietic disorder treatment in alimentary toxic aleukia
Cell Biology and Toxicology (2023)
-
Exposure to hypoxia causes stress erythropoiesis and downregulates immune response genes in spleen of mice
BMC Genomics (2021)
-
Human erythrocytes: cytoskeleton and its origin
Cellular and Molecular Life Sciences (2020)
-
Sphingolipid-mediated inflammatory signaling leading to autophagy inhibition converts erythropoiesis to myelopoiesis in human hematopoietic stem/progenitor cells
Cell Death & Differentiation (2019)
-
Bone marrow hematopoietic dysfunction in untreated chronic lymphocytic leukemia patients
Leukemia (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.