Abstract
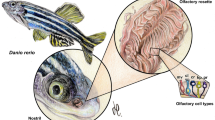
The key behavioural, physiological and anatomical components of a magnetite-based magnetic sense have been demonstrated in rainbow trout (Oncorhynchus mykiss )1. Candidate receptor cells located within a discrete sub-layer of the olfactory lamellae contained iron-rich crystals that were similar in size and shape to magnetite crystals extracted from salmon1,2. Here we show that these crystals, which mapped to individual receptors using confocal and atomic force microscopy, are magnetic, as they are uniquely associated with dipoles detected by magnetic force microscopy. Analysis of their magnetic properties identifies the crystals as single-domain magnetite. In addition, three-dimensional reconstruction of the candidate receptors using confocal and atomic force microscopy imaging confirm that several magnetic crystals are arranged in a chain of about 1 µm within the receptor, and that the receptor is a multi-lobed single cell. These results are consistent with a magnetite-based detection mechanism2,3, as 1-µm chains of single-domain magnetite crystals are highly suitable for the behavioural and physiological responses to magnetic intensity previously reported in the trout.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Walker, M. M. et al. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376 ( 1997).
Kirschvink, J. L. & Walker, M. M. in Magnetite Biomineralization and Magnetoreception by Living Organisms: A New Biomagnetism (eds Kirschvink, J. L., Jones, D. S. & MacFadden, B. J.) 243–254 (Plenum, New York, 1985).
Kirschvink, J. L. & Gould, J. L. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems 13, 181–201 ( 1981).
Yorke, E. D. A possible magnetic transducer in birds. J. Theor. Biol. 77, 101–105 (1979).
Yorke, E. D. Sensitivity of pigeons to small magnetic field variations. J. theor. Biol. 89, 533–537 ( 1981).
Gould, J. L. Magnetic field sensitivity in animals. Ann. Rev. Physiol. 46, 585–598 (1984).
Kirschvink, J. L. Biogenic ferrimagnetism: A new biomagnetism, in Biomagnetism (eds Williamson, S. J., Romani, G. L., Kaufman, L. & Modena, I.) 501–531 (Plenum, New York, 1983).
Proksch, R. B. et al. Magnetic force microscopy of the submicron magnetic assembly in a magnetotactic bacterium. Appl. Phys. Lett. 66, 2582–2584 (1995).
Babcock, K., Dugas, M., Manalis, S. & Elings, V. Magnetic force microscopy. Res. Soc. Symp. Proc. 355, 311 (1995).
Wittborn, J. et al. Magnetization reversal observation and manipulation of chains of nanoscale magnetic particles using the magnetic force microscope. Nano. Mater. 12, 1149–1152 (1999).
Dunin-Borkowski, R. E. et al. Magnetic microsctructure of magnetotactic bacteria by electron holography. Science 282, 1868– 1870 (1998).
Walker, M. M., Quinn, T. P., Kirschvink, J. L. & Groot, C. Production of single-domain magnetite throughout life by sockeye salmon, Oncorhynchus nerka. J. Exp. Biol. 140, 51–63 (1988).
Mann, S., Sparks, N. H. C., Walker, M. M. & Kirschvink, J. L. Ultrastructure, morphology and organization of biogenic magnetite from sockeye salmon, Oncorhynchus nerka; implications for magnetoreception. J. Exp. Biol. 140, 35–49 (1988).
Diaz-Ricci, J. C. & Kirschvink, J. L. Magnetic domain state and coercivity predictions for biogenic greigite (Fe3S 4): A comparison of theory with magnetosome observations. J. Geophys. Res. 97, 17039–17315 (1992).
Semm, P. & Beason, R. C. Responses to small magnetic field variations by the trigeminal system of the bobolink. Brain Res. Bull. 25, 735–740 ( 1990).
Walker, M. M. Learned magnetic field discrimination in the yellowfin tuna, Thunnus albacares . J. Comp. Physiol. A 155, 673– 679 (1984).
Walker, M. M. & Bitterman, M. E. Honeybees can be trained to respond to very small changes in geomagnetic field intensity. J. Exp. Biol. 145, 489–494 (1989).
Wiltschko, R. & Wiltschko, W. Magnetic Orientation in Animals. (Springer, Berlin, Heidelberg, New York, 1995).
Gould, J. L., Kirschvink, J. L., Deffeyes, K. S. Bees have magnetic remanence. Science 201, 1026–1028 ( 1978).
Lohmann, K. J. Magnetic remanence in the Western Atlantic spiny lobster. J. Exp. Biol. 113, 29–41 ( 1984).
Walker, M. M. On a wing and a vector: A model for magnetic navigation by homing pigeons. J. Theor. Biol. 192, 341– 349 (1998).
Walker, M. M. Magnetic position determination by homing pigeons. J. Theor. Biol. 197, 271–276 ( 1999).
Proksch, R. B., Runge, E., Hansma, P. K., Foss, S. & Walsh, B. High field magnetic force microscopy. J. Appl. Phys. 78, 3303–3307 (1995).
Liou, S. H. & Yao, Y. D. Development of high coercivity magnetic force microscopy tips. J. Magn. Magn. Mater. 190, 130–134 (1998).
Acknowledgements
We thank K. Babcock at Digital Imaging for the generous use of the AFM/MFM. The Biological Imaging Research Unit at the School of Medicine, University of Auckland, provided the CLSM and imaging facilities. In addition, we thank S. Edgar, A. Turner, H. Holloway and especially B. Beaumont for their assistance in preparation and viewing samples on the CLSM and transmission electron microscopy. Financial support came from the Marsden Fund and the School of Biological Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diebel, C., Proksch, R., Green, C. et al. Magnetite defines a vertebrate magnetoreceptor. Nature 406, 299–302 (2000). https://doi.org/10.1038/35018561
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35018561
This article is cited by
-
Prussian blue technique is prone to yield false negative results in magnetoreception research
Scientific Reports (2022)
-
Magnetoreception and magnetic navigation in fishes: a half century of discovery
Journal of Comparative Physiology A (2022)
-
Chemical compass behaviour at microtesla magnetic fields strengthens the radical pair hypothesis of avian magnetoreception
Nature Communications (2019)
-
Detection of biogenic magnetic nanoparticles in ethmoid bones of migratory and non-migratory fishes
SN Applied Sciences (2019)
-
Sub-cellular In-situ Characterization of Ferritin(iron) in a Rodent Model of Spinal Cord Injury
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.