Abstract
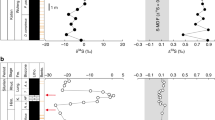
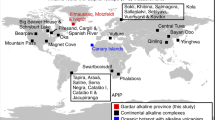
The variation of δ18O that results from nearly all physical, biological and chemical processes on the Earth is approximately twice as large as the variation of δ17O. This so-called ‘mass-dependent’ fractionation is well documented in terrestrial minerals1,2. Evidence for ‘mass-independent’ fractionation (Δ17O = δ17O - 0.52δ18O), where deviation from this tight relationship occurs, has so far been found only in meteoritic material and a few terrestrial atmospheric substances3. In the rock record it is thought that oxygen isotopes have followed a mass-dependent relationship for at least the past 3.7 billion years (ref. 4), and no exception to this has been encountered for terrestrial solids5. Here, however, we report oxygen-isotope values of two massive sulphate mineral deposits, which formed in surface environments on the Earth but show large isotopic anomalies (Δ17O up to 4.6‰). These massive sulphate deposits are gypcretes from the central Namib Desert and the sulphate-bearing Miocene volcanic ash-beds in North America. The source of this isotope anomaly might be related to sulphur oxidation reactions in the atmosphere and therefore enable tracing of such oxidation. These findings also support the possibility of a chemical origin of variable isotope anomalies on other planets, such as Mars6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Clayton, R. N., Grossman, L. & Mayeda, T. K. A component of primitive nuclear composition in carbonaceous chondrites. Science 182, 485– 488 (1973).
Matsuhisa, Y., Goldsmith, J. R. & Clayton, R. N. Mechanisms of hydrothermal crystallization of quartz at 250 degrees C and 15 kilobars. Geochim. Cosmochim. Acta 42, 173–182 (1978).
Thiemens, M. H. Atmosphere science—Mass-independent isotope effects in planetary atmospheres and the early solar system. Science 283, 341–345 (1999).
Robert, F., Rejou-Michel, A. & Javoy, M. Oxygen isotropic homogeneity of the Earth: new evidence. Earth Planet. Sci. Lett. 108, 1– 9 (1992).
Clayton, R. N. & Mayeda, T. K. Oxygen isotope studies of achondrites. Geochim. Cosmochim. Acta 60, 1999– 2017 (1996).
Farquhar, J., Thiemens, M. H. & Jackson, T. Atmosphere-surface interactions on Mars: Delta O-17 measurements of carbonate from ALH 84001. Science 280 , 1580–1582 (1998).
Mauersberger, K. Ozone isotope measurements in the stratosphere. Geophys. Res. Lett. 14, 80–83 ( 1987).
Krankowsky, D., Bartecki, F., Klees, G. G., Mauersberger, K. & Schellenbach, K. Measurement of heavy isotope enrichment in tropospheric ozone. Geophys. Res. Lett. 22, 1713–1716 (1995).
Johnston, J. C. & Thiemens, M. H. The isotopic composition of tropospheric ozone in three environments. J. Geophys. Res. 102 (D21), 25395–25404 (1997).
Savarino, J. & Thiemens, M. H. Analytical procedure to determine both delta O-18 and delta O-17 of H2O2 in natural water and first measurements. Atmos. Environ. 33, 3683–3690 (1999).
Lee, C. W. Multiple stable oxygen isotopic studies of atmospheric sulfate aerosols. Am. Geophys. Union 78, F111 ( 1997).
Lee, C. W., Savarino, J. & Thiemens, M. H. Multiple stable oxygen isotopic studies of sulfate and hydrogen peroxide collected from rain water: a new way to investigate in-situ S(IV) oxidation chemistry by dissolved H2O2 in aqueous solution. Am. Geophys. Union 79, F91 (1998).
Eckardt, F. D. & Spiro, B. The origin of sulphur in gypsum and dissolved sulphate in the Central Namib Desert, Namibia. Sedim. Geol. 123, 255–273 (1999).
Siesser, W. G. Late Miocene origin of the Benguela upwelling system off northern Namibia. Science 208, 283–285 (1980).
Armstrong, R. L. & Ward, P. L. Evolving geographic patterns of Cenozoic magmatism in the North American Cordillera; the temporal and spatial association of magmatism and metamorphic core complexes Mid-Tertiary Cordilleran magmatism; plate convergence versus intraplate processes. J. Geophys. Res. B 96, 13201–13224 (1991).
Nicknish, J. M. & Macdonald, J. R. Basal Miocene ash in White River Badlands, South Dakota. Bull. Am. Assoc. Petrol. Geol. 46, 685–690 ( 1962).
Swisher, C. C. III . Stratigraphy And Biostratigraphy Of The Eastern Portion Of Wildcat Ridge, Western Nebraska. Thesis, Univ. Nebraska ( 1982).
Rose, W. I. Jr, Chuan, R. L., Cadle, R. D. & Woods, D. C. Small particles in volcanic eruption clouds. Am. J. Sci. 280, 671–696 (1980).
Gamsjäger, H. & Murmann, R. K. in Advances in Inorganic and Bioinorganic Mechanisms (ed. Sykes, A. G.) 317 –381 (Academic, London, 1983).
Cerling, T. E. Carbon dioxide in the atmosphere – evidence from Cenozoic and Mesozoic paleosols. Am. J. Sci. 291, 377– 400 (1991).
Rye, R. & Holland, H. D. Paleosols and the evolution of atmospheric oxygen: A critical review. Am. J. Sci. 298, 621–672 (1998).
Berner, R. A. et al. Isotope fractionation and atmospheric oxygen: Implications for phanerozoic O2 evolution. Science 287 , 1630–1633 (2000).
Karlsson, H. R., Clayton, R. N., Gibson, E. K. & Mayeda, T. K. Water in SNC meteorites - Evidence for a martian hydrosphere. Science 255, 1409–1411 ( 1992).
Farquhar, J. & Thiemens, M. H. The oxygen cycle of the Martian atmosphere-regolith system: Δ17O of secondary phases in Nakhla and Lafayette. J. Geophys. Res. 105 (E5), 11991–11997 (2000).
Bao, H. & Thiemens, M. H. Generation of O2 from BaSO4 using a CO2-laser fluorination system for simultaneous δ18O and δ17O analysis. Anal. Chem. (in the press).
Clayton, R. N. & Mayeda, T. K. Oxygen isotopes in eucrites, shergottites, nakhlites, and chassignites. Earth Planet. Sci. Lett. 62, 1–6 ( 1983).
Bhattacharya, S. K. & Thiemens, M. H. New evidence for symmetry dependent isotope effects - O+CO reaction. Z. Naturforsch. A J. Phys. Sci. 44, 435–444 (1989).
Forrest, J. & Newman, L. Silver-110 microgram sulfate analysis for the short time resolution of ambient level of sulfur aerosol. Anal. Chem. 49, 1579–1584 (1977).
Acknowledgements
We thank T. Jackson for technical assistance, J. Cannia for sand samples from Scotts Bluff, Nebraska, J. Alt for marine ferric oxide samples, J. Savarino for helpful discussions, and NASA and NSF for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, H., Thiemens, M., Farquhar, J. et al. Anomalous 17O compositions in massive sulphate deposits on the Earth. Nature 406, 176–178 (2000). https://doi.org/10.1038/35018052
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35018052
This article is cited by
-
Sulfate triple-oxygen-isotope evidence confirming oceanic oxygenation 570 million years ago
Nature Communications (2023)
-
The Isotopic Imprint of Life on an Evolving Planet
Space Science Reviews (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.