Abstract
Cell transfection is now a central technique in molecular biology and an essential prerequisite for gene therapy. Here we describe how beads coated with antibodies and bound to specific cell-surface transmembrane proteins can create holes in cells when the beads are removed, allowing transfection of the cells with DNA or other macromolecules. This unique targeted transfection of cells by immunoporation is very efficient and results in minimal cell death.
Similar content being viewed by others
Main
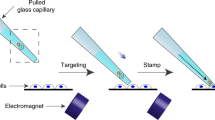
A variety of methods have been developed for the transfection of cells, including electroporation1,2, lipofection3,4, calcium phosphate coprecipitation5,6 and DEAE dextran7,8. Of these methods, only electroporation offers the possibility of introducing DNA and other molecules such as proteins into viable cells. None of the current methods is able to target specific types of cells for transfection. In this new method of cell transfection, antibody-coated beads are bound to specific surface antigens and then the beads are sheared off from the cell by mixing: this causes the formation of transient holes in the cell membrane through which macromolecules can enter.
Granulocytic, differentiating human lymphoblastic HL-60 cells normally express CD71 on their surfaces. When induced to differentiate in the presence of dimethyl sulphoxide (DMSO), the cells cease to express CD71 and instead express CD11b. We have used this cell line as a model system to investigate the process of cell transfection mediated by immunoporation.
DYNAFECT beads coated with either anti-CD11b antibody (DYNAFECT-CD11b) or anti-CD71 antibody (DYNAFECT-CD71) were mixed on a rotating end-over-end mixer at 33 r.p.m. for 6 h at 22 °C with either uninduced HL-60 cells or cells that had been induced with DMSO for 3 days. For mixing in a 2-ml microcentrifuge tube, 107 beads and 5×105 cells were suspended in 0.5 ml transfection medium (Dynal AS) containing 0.2 μg plasmid DNA vector pEGFP-C1 (4.7 kilobases) which codes for green fluorescent protein. After transfection, the beads were removed using a magnetic separator and the cells were transferred back into tissue-culture medium and cultured for a further 48 hours before analysis.
The extent of cell transfection was determined by flow cytometry. Figure 1 shows that the DYNAFECT-CD71 beads facilitate the transfection of DNA into normal HL-60 cells, but when the cells become differentiated and no longer express CD71, transfection no longer occurs with these beads. In contrast, mixing undifferentiated HL-60 cells with DYNAFECT-CD11b beads does not result in the transfection of the cells with DNA, but when the cells are differentiated and begin to express CD11b, those beads do bring about transfection.
Hence, immunoporation has the potential to target specific types of cell in a mixed population for transfection, depending on their immunological identity, and allow the targeted cells to take up a variety of different molecules. This also occurs in several mammalian cell lines with a range of different antibodies that target selected cell-surface antigens. In all cases, the level of transfection was 40–80%, depending on mixing conditions, and non-viable cells usually numbered less than 20%.
The high levels of selectivity and transfection, together with minimal cell death, that are achievable with immunoporation illustrate the enormous potential of this technique for use in a wide range of transfection studies. In particular, the ability to target specific subpopulations of cells will be extremely useful for many gene therapy applications.
References
Fromm, M., Callis, J., Taylor, L. P. & Walbot, V. Methods Enzymol. 153, 351 (1987 ).
Pfau, J. & Youderian, P. Nucleic Acids Res. 18, 6165 (1990).
Watanabe, Y. et al. J. Biochem. 116, 1220– 1226 (1994).
Hargest, R., Eldin, A. & Williamson, R. Adv. Exp. Med. Biol. 451, 385 –391 (1998).
Ambrosini, E. et al. J. Neurosci. Res. 55, 569– 577 (1999).
Nielson, T. et al. Mol. Gen. Genet. 242, 280– 288 (1994).
Muck, K. D., Wei, R. & Elbagarri, A. J. Immunol. Methods 211, 79– 86 (1998).
Yang, Y. W. & Yang, J. C. Biotechnol. Appl. Biochem. 25, 47–51 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bildirici, L., Smith, P., Tzavelas, C. et al. Transfection of cells by immunoporation. Nature 405, 298 (2000). https://doi.org/10.1038/35012701
Issue Date:
DOI: https://doi.org/10.1038/35012701
This article is cited by
-
Nanomagnetic Activation as a Way to Control the Efficacy of Nucleic Acid Delivery
Pharmaceutical Research (2015)
-
Clusterin/Apolipoprotein J up-regulation after zinc exposure, replicative senescence or differentiation of human haematopoietic cells
Biogerontology (2006)
-
Targeted transfection by femtosecond laser
Nature (2002)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.