Abstract
Parental exposure to ionizing radiation increases the frequency of germline mutations detectable in the next generation1. Parental exposure can also increase the rate of mutation in somatic cells2,3 and confer a predisposition to cancer4,5,6 in offspring, suggesting that there could be an indirect effect of radiation on somatic genome stability that is transmissible through the germ line of the irradiated parents. We have found that this indirect effect extends to the germ line of unexposed first-generation offspring in mice, as revealed by an increased instability of repeat-DNA sequences in their descendants.
Similar content being viewed by others
Main
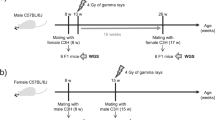
CBA/H male mice were exposed to fission neutrons and mated to unexposed females (Fig. 1a). Exposure resulted in a 6-fold increase in paternal mutation rate at expanded simple-tandem repeat (ESTR) loci7,8 detectable in first-generation (F1) offspring (Table 1). Breeding from these unexposed F1 mice revealed that germline mutation rates remained significantly high in transmissions from both F1 males and F1 females (6-fold and 3.5-fold increase, respectively, compared to unexposed control families and to the alleles transmitted from control breeding partners; not shown).
a, Design of the transgenerational study: the number of F2 offspring derived from each F1 mouse is indicated. b , Frequency of ESTR mutation in offspring of F0 and F1 males and females. Four CBA/H males were given whole-body chronic irradiation of 0.5 Gy of fission neutrons (252Cf source, 0.03 Gy min−1) and 10 weeks later mated to non-irradiated CBA/H females, ensuring that litters were derived from irradiated stem cells. Litters from one irradiated male were used for subsequent breeding of F2 mice. DNA profiles were produced9 using the mouse single-locus ESTR probes Ms6-hm and Hm-2 (refs 7,8).
This increase was in part due to increased mutational mosaicism in the germ line of the F1 mice, with more than 50% of de novo mutations detected in F2 mice being shared by two or more littermates, compared with less than 25% in the offspring of both non-irradiated mice and F0 mice exposed to X-rays9 or fission neutrons (Table 1 and Fig. 1b). However, singleton mutations (those present in only one member of a litter) were also more frequent in these F2 mice than in the controls, significantly so for male transmissions (Table 1). We conclude that paternal exposure to radiation results in the destabilization of ESTR loci in the germ line of offspring, and that some of the resulting mutations occur sufficiently early in germline development for significant levels of mosaicism to arise.
This remarkable stimulation of mutation in the F1 germ line following paternal irradiation is reminiscent of delayed radiation-induced genome instability in somatic cells, where radiation can induce genetic changes in some of the descendants of a single irradiated cell, an effect that can persist for many cell generations10. By analogy, exposure of F0 mice must create a signal in the F0 germ line that is transmitted by a single sperm to the F1 offspring, in which instability arises many cell generations later and is detected in some but not all cells in the developing germ line.
It is unlikely that the negligible cytoplasmic component of the mature sperm could carry substantial amounts of long-lived free radicals or other radiation-induced species into the egg, so the transmitted signal is probably DNA-dependent. It is most unlikely that this signal is radiation-induced damage at the ESTR loci themselves, because it is transmissible through meiosis and mitosis and appears to operate in trans in the F1 germ line, affecting alleles both from the exposed F0 male and from the unexposed F0 female (data not shown).
Increased ESTR mutation rates appear to be uniform in the germ line of all F1 offspring derived from 10 independent sperm from the irradiated F0 male (Fig. 1b; χ2 test for homogeneity, P > 0.5), suggesting that radiation-induced de novo mutations in DNA-repair genes are not the cause of instability in the F1 germ line. The alternative, therefore, is a radiation-exposure signal inherited through sperm in an epigenetic fashion, perhaps through changes in DNA methylation. Methylation is transmissible through many cell divisions11 and can influence the activity of DNA-repair systems12. If radiation-induced DNA damage in the germ line triggers an epigenetic signal that influences DNA repair and can survive the reprogramming of DNA methylation during spermatogenesis and early development, then subsequent activation of this signal in the germ line could lead to the observed transgenerational mutagenesis.
These findings have potential implications for risk evaluation in humans. So far, the main concern of radiation protection is focused on the direct mutagenic effects of ionizing radiation in exposed individuals1. However, the persistence of instability into the germ line of unexposed offspring of irradiated mice raises the issue of delayed genetic risk, as well as the potential for radiation to cause mutation clustering and to contribute to mosaicism in germ cells, long recognized as an important mechanism in the origin of human genetic disorders13.
References
UNSCEAR Sources and Effects of Ionizing Radiation (United Nations, New York, 1993).
Luke, G. A., Riches, A. C. & Bryant, P. E. Mutagenesis 12, 147– 152 (1997).
Burruel, V. R., Raabe, O. G. & Wiley, L. M. Mutat. Res. 381, 59– 66 (1997).
Nomura, T. Mutat. Res. 121, 59–68 ( 1983).
Vorobtsova, I. A., Aliyakparova, C. M. & Anisimov, V. N. Mutat. Res. 287, 207– 216 (1993).
Lord, B. I. et al. Br. J. Cancer 78, 301– 311 (1998).
Kelly, R. et al. Genomics 5, 844–856 (1989).
Gibbs, M. et al. Genomics 17, 121–128 (1993).
Dubrova, Y. E. et al. Proc. Natl Acad. Sci. USA 95, 6251– 6255 (1998).
Morgan, W. F. et al. Radiat. Res. 146, 247– 258 (1996).
Holliday, R. Science 238, 163–170 ( 1987).
Herman, J. G. et al. Proc. Natl Acad. Sci. USA 95, 6870– 6875 (1998).
Zlatogora, J. Hum. Genet. 102, 381–386 (1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubrova, Y., Plumb, M., Gutierrez, B. et al. Transgenerational mutation by radiation . Nature 405, 37 (2000). https://doi.org/10.1038/35011135
Issue Date:
DOI: https://doi.org/10.1038/35011135
This article is cited by
-
Small de novo CNVs as biomarkers of parental exposure to low doses of ionizing radiation of caesium-137
Scientific Reports (2018)
-
Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health
Clinical Epigenetics (2015)
-
Cancer drugs affect mouse genomes for generations
Nature (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.