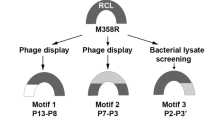
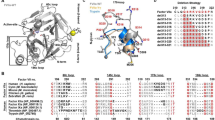
Abstract
Potent anticoagulants have been derived by targeting the tissue factor–factor VIIa complex with naive peptide libraries displayed on M13 phage. The peptides specifically block the activation of factor X with a median inhibitory concentration of 1 nM and selectively inhibit tissue-factor-dependent clotting. The peptides do not bind to the active site of factor VIIa; rather, they work by binding to an exosite on the factor VIIa protease domain, and non-competitively inhibit activation of factor X and amidolytic activity. One such peptide (E-76) has a well defined structure in solution determined by NMR spectroscopy that is similar to the X-ray crystal structure when complexed with factor VIIa. These structural and functional studies indicate an allosteric ‘switch’ mechanism of inhibition involving an activation loop of factor VIIa and represent a new framework for developing inhibitors of serine proteases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nemerson, Y. Tissue factor and hemostasis. Blood 71, 1–8 (1988).
Rapaport, S. I. & Rao, L. V. M. The tissue factor pathway: How it has become a “prima ballerina”. Thromb. Haemost. 74, 7–17 ( 1995).
Davie, E. W., Fujikawa, K. & Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 30, 10363– 10370 (1991).
Hirsh, J. & Weitz, J. I. Thrombosis: New antithrombotic agents. Lancet 353, 1431– 1436 (1999).
Vlasuk, G. P. The new anticoagulants: New opportunities, new issues. Arch. Pathol. Lab. Med. 122, 812–814 ( 1998).
Hirsh, J., Ginsberg, J. S. & Marder, V. J. in Hemostasis and Thrombosis: Basic Principles and Clinical Practice (eds Colman, R. W., Hirsh, J., Marder, V. J. & Salzman, E. W.) 1567–1583 (J. B. Lippincott, Philadelphia, 1994).
Weitz, J. I. Low molecular weight heparins. N. Engl. J. Med. 337 , 688–698 (1997).
Hirsh, J. Heparin. N. Engl. J. Med. 324, 1565– 1574 (1991).
Broze, G. J. Jr, Girard, T. J. & Novotny, W. F. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry 29, 7539– 7546 (1990).
Broze, G. J. Jr, Likert, K. & Higuchi, D. Inhibition of Factor VIIa/tissue factor by antithrombin III and tissue factor pathway inhibitor. Blood 82, 1679–1680 (1993).
Mann, K. G. Inhibition of Factor VIIa/tissue factor by antithrombin III and tissue factor pathway inhibitor. Blood 82, 1680– 1681 (1993).
Bode, W. & Huber, R. Natural protein proteinase inhibitors and their interaction with proteinases. Eur. J. Biochem. 204, 433–451 (1992).
Laskowski, M. Jr & Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 49, 593–626 (1980).
Sanderson, P. E. J. Small, noncovalent serine protease inhibitors. Med. Res. Rev. 19, 179–197 ( 1999).
Stubbs, M. T. & Bode, W. Coagulation factors and their inhibitors. Curr. Opin. Struct. Biol. 4, 823– 832 (1994).
Bode, W., Brandstetter, H., Mather, T. & Stubbs, M. T. Comparative analysis of haemostatic proteinases: Structural aspects of thrombin, Factor Xa, Factor IXa, and Protein C. Thromb. Haemost. 78, 501–511 (1997).
Kirchhofer, D. & Banner, D. W. Molecular and structural advances in tissue factor-dependent coagulation. Trends Cardiovasc. Med. 7, 316–324 (1997).
Ruf, W. & Dickinson, C. D. Allosteric regulation of the cofactor-dependent serine protease coagulation Factor VIIa. Trends Cardiovasc. Med. 8, 350–356 (1998).
Higashi, S. & Iwanaga, S. Molecular interaction between factor VII and tissue factor. Int. J. Hematol. 67, 229–241 (1998).
Banner, D. W. et al. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature 380, 41–46 (1996).
Johnson, K. & Hung, D. Novel anticoagulants based on inhibition of the factor VIIa/tissue factor pathway. Coron. Artery Dis. 9, 83–87 (1998).
Lowman, H. B. Bacteriophage display and discovery of peptide leads for drug development. Annu. Rev. Biophys. Biomol. Struct. 26, 401–424 (1997).
Lowman, H. B. et al. Molecular mimics of insulin-like growth factor 1 (IGF-1) for inhibiting IGF-1: IGF-binding protein interactions. Biochemistry 37, 8870–8878 ( 1998).
Dennis, M. S. & Lazarus, R. A. Kunitz domain inhibitors of tissue factor-Factor VIIa I. Potent inhibitors selected from libraries by phage display. J. Biol. Chem. 269, 22129– 22136 (1994).
Segel, I. H. in Enzyme Kinetics 100–226 (Wiley, New York, 1975).
Neuenschwander, P. F., Branam, D. E. & Morrissey, J. H. Importance of substrate composition, pH, and other variables on tissue factor enhancement of Factor VIIa activity. Thromb. Haemost. 70, 970–977 (1993).
Zhang, E., St. Charles, R. & Tulinsky, A. Structure of extracellular tissue factor complexed with Factor VIIa inhibited with a BPTI mutant. J. Mol. Biol. 285, 2089–2104 (1999).
Pike, A. C. W., Brzozowski, A. M., Roberts, S. M., Olsen, O. H. & Persson, E. Structure of human factor VIIa and its implications for the triggering of blood coagulation. Proc. Natl Acad. Sci. USA 96, 8925–8930 (1999).
Kraut, J. Serine proteases: Structure and mechanism of catalysis. Annu. Rev. Biochem. 46, 331–358 ( 1977).
Steitz, T. A. & Shulman, R. G. Crystallographic and NMR studies of the serine proteases. Annu. Rev. Biophys. Bioeng. 11, 419–444 (1982).
Polgár, L. in Mechanisms of Protease Action 87–122 (CRC, Boca Raton, 1989).
Skrzypczak-Jankun, E. et al. Structure of hirugen and hirulog 1 complexes of alpha-thrombin. J. Mol. Biol. 221, 1379– 1393 (1991).
Stubbs, M. T. & Bode, W. A player of many parts: The spotlight falls on thrombin's structure. Thromb. Res. 69, 1–58 (1993).
Fuentes-Prior, P. et al. Structure of the thrombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug. Proc. Natl Acad. Sci. USA 94, 11845–11850 (1997).
Naski, M. C., Fenton, J. W. I., Maraganore, J. M., Olson, S. T. & Shafer, J. A. The COOH-terminal domain of hirudin. An exosite-directed competitive inhibitor of the action of alpha-thrombin on fibrinogen. J. Biol. Chem. 265, 13484 –13489 (1990).
Shobe, J., Dickinson, C. D. & Ruf, W. Regulation of the catalytic function of coagulation Factor VIIa by a conformational linkage of surface residue Glu 154 to the active site. Biochemistry 38, 2745– 2751 (1999).
Huber, R. & Bode, W. Structural basis of the activation and action of trypsin. Acc. Chem. Res. 11, 114–121 (1978).
Bode, W. & Huber, R. in Molecular and Cellular Basis of Digestion (eds Desnuelle, P., Sjöström, H. & Norén, O.) 213–234 (Elsevier, Amsterdam, 1986).
Gallop, M. A., Barrett, R. W., Dower, W. J., Fodor, S. P. A. & Gordon, E. M. Applications of combinatorial technologies to drug discovery. J. Med. Chem. 37, 1233–1251 (1994).
Kelley, R. F. et al. A soluble tissue factor mutant is a selective anticoagulant and antithrombotic agent. Blood 89, 3219 –3227 (1997).
Skelton, N. J., Garcia, K. C., Goeddel, D. V., Quan, C. & Burnier, J. P. Determination of the solution structure of guanylin. Biochemistry 33, 13581–13592 (1994).
Toomey, J. R., Smith, K. J. & Stafford, D. W. Localization of the human tissue factor recognition determinant of human Factor VIIa. J. Biol. Chem. 266 , 19198–19202 (1991).
Kunkel, T. A., Roberts, J. D. & Zakour, R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154, 367–382 (1987).
Marquardt, D. W. An algorithm for least squares estimation of non linear parameters. J. Soc. Indust. Appl. Math. 11, 431– 441 (1963).
Kemball-Cook, G., Johnson, D. J. D., Tuddenham, E. G. D. & Harlos, K. Crystal structure of active site-inhibited human coagulation factor VIIa (des-Gla). J. Struct. Biol. 127, 213– 223 (1999).
Acknowledgements
We thank R. Kelley for TF and helpful discussions; R. Artis, R. McDowell, C. Refino and D. Kirchhofer for helpful discussions; R. Smyth and S. Bullens for clotting assays; H. Lowman, B. Cunningham and G. Nakamura for help with library constructions; C. Quan, J. Tom and M. Struble for peptide synthesis; M. Beresini, A. Hebert and L. Caris for ELISA assays; W. Prince for inhibition assays; P. Jhurani, P. Ng and M. Vasser for DNA synthesis; M. Hamner, A. Zhong and A. Goddard for DNA sequencing; D. Stafford and J. Toomey for plasmids encoding FVII and FVII/FIX chimaeras; B. J. Clarke for a preprint on rabbit FVIIa expression and characterization; and A. de Vos, J. Burnier and D. Lowe for their support. This work is based in part upon research conducted at the Stanford Synchrotron Radiation Laboratory (SSRL), which is funded by the Department of Energy, Office of Basic Energy Sciences.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dennis, M., Eigenbrot, C., Skelton, N. et al. Peptide exosite inhibitors of factor VIIa as anticoagulants. Nature 404, 465–470 (2000). https://doi.org/10.1038/35006574
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35006574
This article is cited by
-
Allosteric inhibition of HTRA1 activity by a conformational lock mechanism to treat age-related macular degeneration
Nature Communications (2022)
-
Hot spot prediction in protein-protein interactions by an ensemble system
BMC Systems Biology (2018)
-
Beyond Antibodies: Development of a Novel Protein Scaffold Based on Human Chaperonin 10
Scientific Reports (2016)
-
Ringhalexin from Hemachatus haemachatus: A novel inhibitor of extrinsic tenase complex
Scientific Reports (2016)
-
Phage-encoded combinatorial chemical libraries based on bicyclic peptides
Nature Chemical Biology (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.