Abstract
Originally published as Nature 397, 263–266; 1999
The T-cell-specific cell-surface receptors CD28 and CTLA-4 are important regulators of the immune system. CD28 potently enhances those T-cell functions that are essential for an effective antigen-specific immune response1,2,3,4,5, and the homologous CTLA-4 counterbalances the CD28-mediated signals and thus prevents an otherwise fatal overstimulation of the lymphoid system6,7,8,9. Here we report the identification of a third member of this family of molecules, inducible co-stimulator (ICOS), which is a homodimeric protein of relative molecular mass 55,000–60,000 ( Mr 55K–60K). Matching CD28 in potency, ICOS enhances all basic T-cell responses to a foreign antigen, namely proliferation, secretion of lymphokines, upregulation of molecules that mediate cell–cell interaction, and effective help for antibody secretion by B cells. Unlike the constitutively expressed CD28, ICOS has to be de novo induced on the T-cell surface, does not upregulate the production of interleukin-2, but superinduces the synthesis of interleukin-10, a B-cell-differentiation factor. In vivo, ICOS is highly expressed on tonsillar T cells, which are closely associated with B cells in the apical light zone of germinal centres, the site of terminal B-cell maturation. Our results indicate that ICOS is another major regulator of the adaptive immune system.
Similar content being viewed by others
Main
We identified ICOS by generating monoclonal antibodies against activated human T cells. The ICOS-specific monoclonal antibody F44 did not react with resting human peripheral-blood T cells, but stained CD4+ and CD8+ T lymphocytes that had been activated by stimulation of the T-cell antigen receptor (TCR) complex (Fig. 1a). No signal was detected on resting or appropriately activated B cells, monocytes, natural killer cells, granulocytes, dendritic cells or platelets (data not shown). Immunoprecipitations using monoclonal antibody F44 defined the ICOS antigen as a disulphide-linked dimer, with an apparent relative molecular mass of 55K–60K, composed of a 27K and a 29K chain ( Fig. 1b). We enriched ICOS protein from lysates of the ICOS-expressing T-cell line MOLT-4V by large-scale affinity chromatography using F44, and further purified the protein by two-dimensional preparative SDS–polyacrylamide gel electrophoresis (PAGE) (Fig. 1b). The 27K and 29K ICOS-protein species yielded identical peptide sequences, indicating that ICOS may be expressed on the cell surface as a homodimeric protein, with the two chains differing only in their post-translational modification; this assumption was later confirmed by transfection experiments (data not shown).
a, Expression of ICOS on human T cells after 36 h of stimulation. Peripheral-blood CD4+ or CD8+ T cells were left untreated or were stimulated with the solid-phase-bound anti-CD3 monoclonal antibody OKT 3 (1:1,000 dilution of ascites), and were analysed by flow cytometry using the fluorescein isothiocyanate (FITC)-labelled monoclonal antibody F44. At 14 h after stimulation, ICOS could not yet be detected on CD8+ T cells, whereas CD4+ T cells expressed levels of ICOS that were similar to those expressed after 36 h (data not shown). The background signal obtained with the isotype-control monoclonal antibody MOPC-21 (Sigma) is shown in black. Stimulation by phorbol-12-myristate-13-acetate (PMA) and ionomycin led to the expression of ICOS on most CD4+ and CD8+ T cells (data not shown). b, Structure of the homodimeric ICOS protein. ICOS protein was immunoprecipitated from surface-iodinated MOLT-4V cells with monoclonal antibody F44 and separated by two-dimensional (non-reducing/reducing) SDS–PAGE. Numbers at the top and right side of the gel are Mr values. The 55K–60K protein species on the diagonal corresponds to residual dimeric ICOS, which was not reduced by the in-gel reducing procedure required for the two-dimensional SDS–PAGE (a full reduction of this species was routinely observed in one-dimensional SDS–PAGE; data not shown). Identical data were obtained with iodinated activated primary T cells (data not shown). c, ICOS mRNA expression. Amounts of ICOS mRNA were determined by northern blot analysis of total RNA (2.5 µg per lane) obtained from human peripheral-blood CD4+ T cells activated with PMA (20 ng ml−1) and ionomycin (200 ng ml−1) for 24 h. d, Amino-acid-sequence alignment of ICOS and CD28 (ref. 28), obtained using the Clustal-W algorithm with BLOSUM 30 matrix (MacVector, Oxford Molecular Group). Identical amino-acid residues are shaded; conserved residues are boxed; potential N-glycosylation sites are indicated by asterisks. Predicted signal peptides (the exact cleavage site for ICOS has not been determined experimentally), IgV-like domains29 and predicted transmembrane regions are indicated. Cysteine residues that may be important in maintaining three-dimensional structure are shown in black.
Using the peptide sequence, we cloned full-length ICOS complementary DNA (2,641 base pairs) from a MOLT-4V cDNA library. Northern analysis revealed a single ICOS messenger RNA species of ∼2.8 kilobases in length in activated human T cells (Fig. 1c). The open reading frame of ICOS mRNA encodes a new protein of 199 amino acids with a predicted relative molecular mass of 22.6K. The ICOS amino-acid sequence shares 24% (17%) identity and 39% (39%) similarity with CD28 (Fig. 1d) (and CTLA-4 (ref. 10)). The predicted mature ICOS is a type-I transmembrane molecule (type I transmembrane proteins have their carboxy termini in the cytosol) that consists of a single immunoglobulin (Ig)V-like domain (stabilized by conserved cysteine residues at positions 42 and 109; Fig. 1d), a transmembrane region of ∼23 amino acids, and a cytoplasmic tail of 35 amino acids, and it shows a close structural resemblance to CD28 (Fig. 1d) and CTLA-4 (ref. 10). The cysteine residue located at position 141 of CD28, also found in CTLA-4, is apparently involved in forming the disulphide bridge between the homodimeric chains of these proteins, and is also found in ICOS (position 136). The motif MYPPPY (positions 117–122 in CD28), required in its intact form for the binding of CD28 and CTLA-4 to their counter-receptors B7-1 and B7-2 (refs 11, 12 ), is not conserved in ICOS, indicating that ICOS may interact with a different receptor.
In a series of in vitro experiments, we compared ICOS to the main co-stimulatory effects of CD28 directly. We tested the ICOS-specific monoclonal antibody F44 in parallel with monoclonal antibody 9.3, one of the most potent CD28-specific reagents available1,13. When human peripheral-blood CD4+ T cells were suboptimally activated through the CD3 complex of the TCR, co-stimulation of T-cell proliferation by ICOS was comparable in potency to signalling by CD28 (shaded area in upper panel of Fig. 2a). This finding was remarkable, given that ICOS was present on at most 30–40% of the CD4+ T cells under these experimental conditions (shaded area in lower panel of Fig. 2a), whereas CD28 continued to be expressed on nearly all CD4+ T cells. At higher dilutions of the CD3-specific monoclonal antibody OKT 3, the co-stimulatory effect of monoclonal antibody F44 could no longer be achieved, because the signal through the TCR complex was insufficient to induce the expression of the ICOS molecule on the T-cell surface ( Fig. 2a, lower panel).
a, Co-stimulation of T-cell proliferation. Peripheral-blood CD4+ T cells were cultured in the presence of monoclonal antibody OKT 3 (dilutions of ascites), and monoclonal antibodies 9.3 (open circles), F44 (filled circles), or isotype-control MOPC-21 (triangles) (see Methods). Top, proliferation of T cells was determined by addition of 1 µCi [3H]thymidine for the last 16 h of the 72 h culture period. The shaded area shows the experimental point at which maximal co-stimulation with F44 was observed. In ten experiments, co-stimulation through ICOS enhanced proliferation of T cells by 60- to 80-fold (range 14- to 135-fold) over the background stimulation observed with OKT 3 alone. Bottom, the percentage of T cells induced to express ICOS at the various dilutions of OKT 3 ascites in cultures with co-crosslinked F44 at 24 h after stimulation, the time point of maximal ICOS expression. b, Co-stimulation of lymphokine secretion by primary T cells. Peripheral-blood CD4+ cells were stimulated by optimal CD3-crosslinking (1:1,000 dilution of OKT 3 ascites) in the presence of co-stimulating monoclonal antibody 9.3 (white columns) or F44 (black columns), or in the presence of the control monoclonal antibody MOPC-21 (stippled columns). Supernatants were collected at the indicated times after stimulation and assayed for various cytokines using enzyme-linked immunosorbent assays (ELISAs) (Biosource). Results of a representative experiment out of six are shown. c, Upregulation of the cell-surface molecule CD154 (also known as CD40 ligand and TRAP). Peripheral blood CD4+ T cells were suboptimally stimulated (1:5,000 dilution of OKT 3 ascites) in the presence of control monoclonal antibody MOPC-21 or monoclonal antibodies F44 or 9.3, and CD154 expression was determined by flow cytometry with an FITC-labelled antibody, TRAP1 (ref. 15 ). d, Effect of T-cell co-stimulation through ICOS on immunoglobulin synthesis by tonsillar B cells. Peripheral-blood CD4+ T cells were cultured with tonsillar B cells in microtitre plates precoated with monoclonal antibody OKT 3 (1:5,000 dilution of ascites) and MOPC-21, F44 or 9.3. IgM and IgG levels in the culture supernatants were determined by ELISA on day 8. Error bars indicate the s.d. from quadruplicate cultures. Results from a representative experiment out of six are shown.
Only a small amount of most lymphokines is secreted from primary CD4+ T cells after optimal triggering of the CD3/TCR complex alone14. One characteristic of co-stimulation of CD4+ T cells by CD28 is the enhancement of cytokine secretion, in particular the ‘superinduction’ of interleukin (IL)-2 (Fig. 2b and refs 1, 3, 4, 14). Surprisingly, co-stimulation by ICOS failed to significantly upregulate IL-2 at any time point ( Fig. 2b). However, co-stimulation by ICOS upregulated the secretion of IL-4, IL-5, interferon-γ, tumour-necrosis factor-α and granulocyte/macrophage-colony-stimulating factor to 50–70% of the levels achieved with co-stimulation by CD28 (Fig. 2b and data not shown). ICOS and CD28 had markedly different co-stimulatory effects on the secretion of IL-10. In contrast to the modest stimulatory capacity of CD28, co-stimulation by ICOS routinely induced several-fold higher secretion of IL-10 (Fig. 2b); northern blot analysis confirmed the upregulation of expression of IL-10 (data not shown). As the proportion of ICOS+ T cells in the lymphokine-co-stimulation system did not exceed 50% (Fig. 2a, lower panel), on a per-cell basis ICOS matched the ability of CD28 to amplify the secretion of many lymphokines (except for IL-2), and was superior to CD28 in co-inducing IL-10.
T cells communicate with other cells of the immune system by de novo expression of cell-surface molecules, as well as by generating lymphokines. Co-stimulation of T cells through ICOS or CD28 markedly upregulated expression of CD154 (also known as CD40 ligand or TRAP), a molecule required for the crosstalk of T cells with B cells15 (Fig. 2c ), and also other early T-cell-activation antigens such as CD69, CD25 or CD71 (data not shown). Next, we tested whether the ICOS-induced expression of a variety of lymphokines and cell-surface receptors was sufficient for the complex communication of T cells with tonsillar B lymphocytes. Unstimulated T cells, or T cells that had only been preactivated through the TCR, could not induce immunoglobulin synthesis by B cells (Fig. 2d). Only co-stimulation through ICOS or CD28 enabled T cells to provide effective help for B cells to synthesize IgM and IgG (Fig. 2d). On a per-cell basis, co-stimulation through ICOS was as effective as co-stimulation through CD28 in upregulating the expression of cell-surface receptors by T cells or IgG synthesis by B cells.
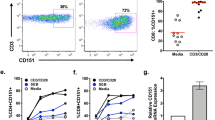
To determine the function of ICOS in vivo, we stained tissue sections of various lymphoid organs with monoclonal antibody F44. In the adult thymus and spleen, ICOS expression was almost completely confined to the few small germinal centres that are present in these tissues (data not shown). In tonsils, a substantial number of cells stained positively for ICOS and we therefore characterized tonsillar cells extensively by flow cytometry. Of these tonsillar T cells, 50–70% expressed high levels of ICOS (Fig. 3a ); all other tonsillar cell populations did not express ICOS (data not shown). The expression of ICOS was associated with the presence of CD28 on the T-cell surface (Fig. 3a). Nearly all ICOS+ T cells also expressed CD45RO (Fig. 3a), indicating that T cells bearing high levels of ICOS may be in a late phase of activation; ICOS− T cells had the phenotype of resting cells (data not shown). Finally, almost all T cells positive for CD57, a marker for a subpopulation of T cells that are located in germinal centres of lymphoid tissues16,17, co-expressed ICOS ( Fig. 3a). Detailed immunohistological studies showed that ICOS is predominantly expressed on germinal-centre T cells located in the apical part of the germinal-centre light zone (Fig. 3b), a site in which T cells are known to induce terminal differentiation of B cells into antibody-secreting plasma cells or memory cells16,17. We also identified a smaller but significant proportion of ICOS+ cells in the T-cell zone surrounding germinal centres (Fig. 3b).
a, Tonsillar T lymphocytes were analysed by three-colour flow cytometry on a FACSCalibur (Becton Dickinson). T cells (5 × 104) were gated with a CD3-specific monoclonal antibody (UCHT1-CyChrome, Pharmingen) and expression of ICOS (monoclonal antibody F44–phycoerythrin) was correlated with the expression of CD28 (CD28.2–FITC), CD45RO (UCHL1–FITC), or CD57 (NC1–FITC; all monoclonal antibodies from Immunotech). Data shown are representative of material obtained from 20 tonsils. b, Localization of ICOS+ cells in the apical light zone of germinal centres, as determined from histochemical stains of frozen human tonsillar sections with monoclonal antibody F44 using the APAAP technique30. LZ, light zone; DZ, dark zone; MZ, mantle zone (original magnification ×69).
On the basis of its structure, its T-cell-restricted expression and its function, we conclude that ICOS is closely related to CD28, an important molecule in the immune system2,3,4,5,16,17. However, despite their overall similarity, ICOS and CD28 differ profoundly in several aspects. In humans, CD28 is constitutively expressed at high levels on almost all CD4+ and on 50% of CD8+ T cells, whereas expression of ICOS must be induced de novo. Both CD28 and ICOS co-induce the synthesis of several lymphokines, but only CD28 superinduces IL-2, whereas ICOS is more effective in superinducing IL-10. It is likely that the interaction of CD28 with its counter-receptors B7-1 and B7-2 is important in the early phases of cooperation between T cells and B cells. At later time points, CTLA-4 counteracts the signals provided by CD28 ( refs 6, 9, 18, 19) and thus terminates the production of IL-2; IL-2 is known to drive the expansion of the germinal-centre B cells17,20,21. ICOS, probably binding to receptor(s) on B cells other than B7-1 and B7-2 (see above), may become the dominant signal for T cells in the later phases of T-cell–B-cell cooperation in the lymphoid system. Such a scenario is probable, because IL-10 can induce the terminal differentiation of B cells into memory cells and antibody-secreting plasma cells22,23,24; this takes place in the apical light zone of the germinal centre16,17, the major site of ICOS expression.
Methods
Cell preparation, T-cell activation and generation of monoclonal antibodies. Peripheral-blood CD4+ (96% pure) and CD8+ (92% pure) cells were negatively purified from buffy coats using nylon wool adherence and magnetobead-coupled (Dynal) monoclonal antibodies specific for CD19, CD11b, major histocompatibility complex (MHC) II molecules, and CD8 or CD4. Peripheral-blood CD4+ or CD8+ T cells (1 × 105 in 200 µl) were activated using the anti-CD3 monoclonal antibody OKT 3 (ATCC; ascites diluted as indicated in Fig. 2a), and were co-stimulated with monoclonal antibody 9.3 (anti-CD28; 1:3,000 dilution of ascites), F44 (anti-ICOS; 4 µg ml−1), or MOPC-21 (IgG1 isotype control; 4 µg ml−1) all of these antibodies were solid-phase-bound through precoating of the 96-well round-bottomed microtitre plates with rabbit anti-mouse serum (10 µg ml−1, Sigma).
To assay cooperation between T and B cells, peripheral-blood CD4+ T cells (50,000 per well) treated with mitomycin C were co-cultured with tonsillar B cells (25,000 per well) in 96-well round-bottomed microtitre plates. Tonsils, obtained from individuals (aged 3–18 yr) undergoing tonsillectomy, were mechanically dispersed and T cells (80–90% pure) were isolated using Ficoll–Hypaque gradient centrifugation followed by nylon-wool adherence. Tonsillar B cells were isolated by rosetting tonsillar cells with sheep red blood cells that had been treated with 2-aminoethyl isothiourea.
Monoclonal antibodies were generated by fusing spleen cells of BALB/c mice that had been immunized with activated human T cells to myeloma P3X63Ag8.653 (ATCC). The hybridoma secreting the ICOS-specific monoclonal antibody F44 (IgG1) was obtained by subcloning of hybridoma 8F4.
Protein purification and sequencing. MOLT-4V cells (a variant of MOLT-4; ATCC) were lysed in 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride (PMSF) and 1% Nonidet P-40 (NP-40) at 4 °C for 1 h (1 ml buffer per 20 × 106 cells ). Nuclei and insoluble material were removed by centrifugation at 1,000 g and 100,000g, respectively. The lysate was preabsorbed with unspecific monoclonal antibody coupled to CnBr-activated Sepharose (Pharmacia) and incubated at 4 °C for 4 h with monoclonal antibody F44 coupled to protein G–Sepharose (Pharmacia) according to ref. 25. The F44 matrix was washed in a column with several volumes of buffer 1 (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 1 mM EDTA, 1 mM PMSF and 0.5% NP-40), buffer 2 (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 0.5% NP-40 and 0.1% SDS), and buffer 3 (0.2 M glycine, pH 4.0, and 0.5% CHAPS), and the bound protein was eluted with two volumes of 0.2 M glycine, pH 2.5, and 0.5% CHAPS. The eluate was concentrated by ultrafiltration (Centricon 10, Amicon) and subjected to preparative two-dimensional non-reducing/reducing gel electrophoresis (20 × 109 cell equivalents per gel). After electrophoresis in the first dimension, the gel area containing the ICOS protein was cut out and subjected to reducing conditions in 5.3 M urea, 0.5 M Tris-HCl, pH 8.0, 1% SDS, 1% β-mercaptoethanol (50 °C for 1 h). Free cysteines were subsequently alkylated by addition of 10 mM iodoacetamide (37 °C for 30 min). After equilibration in 1× SDS–PAGE sample buffer (30 min), the gel piece was mounted onto a SDS–PAGE slab gel (12% polyacrylamide gel). Following electrophoresis, Coomassie-blue-stained spots corresponding to the ICOS protein (Fig. 2b) were cut out. Protein material from five gels was pooled, in situ-digested with trypsin26, fractionated by reverse-phase HPLC on a microRPC C2/C18 SC column (Smart system, Pharmacia) using a linear gradient of acetonitrile and 0.1% trifluoroacetic acid, and sequenced on a pulsed-liquid gas-phase sequencer (Applied Biosystems).
cDNA cloning. Poly(A)+ MOLT-4V RNA was converted to cDNA using oligo-dT primers and Superscript II reverse transcriptase (Gibco), and the cDNA was cloned into λZAPII vector (Stratagene) according to the manufacturer's instructions. Peptide XRLTDVT, obtained from protein microsequencing, was used to derive the two degenerate oligonucleotides MGNCTSACNGAYGTNAC (512 permutations) and MGNYTDACNGAYGTNAC (1,024 permutations) for screening of the generated cDNA library by hybridization in 3 M tetramethyl ammonium chloride27. Several positive clones were sequenced using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems). One of the cDNA clones encoded the above peptide sequence (XRLTDVT) and was used to isolate a full-length cDNA clone.
References
Hara, T., Fu, S. M. & Hansen, J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J. Exp. Med. 161, 1513–1524 (1985).
Shahinian, A. et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261, 609–612 (1993).
Lucas, P. J., Negishi, I., Nakayama, K., Fields, L. E. & Loh, D. Y. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J. Immunol. 154, 5757–5768 (1995).
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Chambers, C. A. & Allison, J. P. Co-stimulation in T cell responses. Curr. Opin. Immunol. 9, 396–404 (1997).
Walunas, T. L. et al . CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 (1994).
Tivol, E. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541– 547 (1995).
Waterhouse, P. et al . Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985– 988 (1995).
Thompson, C. B. & Allison, J. P. The emerging role of CTLA-4 as an immune attenuator. Immunity 7, 445–450 (1997).
Brunet, J. F. et al . Anew member of the immunoglobulin superfamily — CTLA-4. Nature 328, 267–270 (1987).
Peach, R. J. et al. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J. Exp. Med. 180, 2049–2058 ( 1994).
Truneh, A. et al. Differential recognition by CD28 of its cognate counter receptors CD80 (B7.1) and B70 (B7.2): analysis by site directed mutagenesis. Mol. Immunol. 33, 321–334 (1996).
Nunes, J. et al. CD28 mAbs with distinct binding properties differ in their ability to induce T cell activation: analysis of early and late activation events. Int. Immunol. 5, 311–315 (1993).
Thompson, C. B. et al . CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl Acad. Sci. USA 86, 1333–1337 ( 1989).
Kroczek, R. A. et al . Defective expression of CD40 ligand on T cells causes “X-linked immunodeficiency with hyper-IgM (HIGM1)”. Immunol. Rev. 138, 39–59 (1994).
MacLennan, I. C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Tsiagbe, V. K., Inghirami, G. & Thorbecke, G. J. The physiology of germinal centers. Crit. Rev. Immunol. 16, 381–421 (1996).
Krummel, M. F. & Allison, J. P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183, 2533– 2540 (1996).
Walunas, T. L., Bakker, C. Y. & Bluestone, J. A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183, 2541– 2550 (1996).
Nakanishi, K. et al . Both interleukin 2 and a second T cell-derived factor in EL-4 supernatant have activity as differentiation factors in IgM synthesis. J. Exp. Med. 160, 1605– 1621 (1984).
Jung, L. K., Hara, T. & Fu, S. M. Detection and functional studies of p60-65 (Tac antigen) on activated human B cells. J. Exp. Med. 160, 1597– 1602 (1984).
Rousset, F. et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl Acad. Sci. USA 89, 1890–1893 (1992).
Kindler, V. & Zubler, R. H. Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J. Immunol. 159, 2085– 2090 (1997).
Choe, J. & Choi, Y. S. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur. J. Immunol. 28, 508–515 (1998).
Schneider, C., Newman, R. A., Sutherland, D. R., Asser, U. & Greaves, M. F. Aone-step purification of membrane proteins using a high efficiency immunomatrix. J. Biol. Chem. 257, 10766–10769 (1982).
Rosenfeld, J., Capdevielle, J., Guillemot, J. C. & Ferrara, P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 203, 173–179 (1992).
Jacobs, K. A. et al . The thermal stability of oligonucleotide duplexes is sequence independent in tetraalkylammonium salt solutions: application to identifying recombinant DNA clones. Nucleic Acids Res. 16, 4637–4650 (1988).
Aruffo, A. & Seed, B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc. Natl Acad. Sci. USA 84, 8573–8577 ( 1987).
Williams, A. F. & Barclay, A. N. The immunoglobulin superfamily — domains for cell surface recognition. Annu. Rev. Immunol. 6, 381–405 (1988).
Cordell, J. L. et al . Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J. Histochem. Cytochem. 32, 219–229 (1984).
Prediction of transmembrane helices in proteins [online] (cited 01 Dec. 98)〈www.cbs.dtu.dk/services/TMHMM-1.0/〉 ((1998)).
Acknowledgements
We thank J. Ledbetter for the gift of monoclonal antibody 9.3; J. Slupsky for critical reading of the manuscript; and K. Ranke for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hutloff, A., Dittrich, A., Beier, K. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 402 (Suppl 6763), 21–24 (1999). https://doi.org/10.1038/35005523
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35005523
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.