Abstract
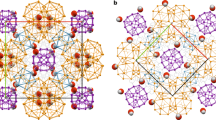
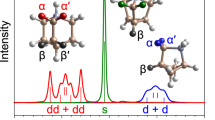
Crystalline supramolecular aggregates consisting of charged organic molecules, held together through metal-cluster-mediated Coulomb interactions, have attracted interest owing to their unusual structural, chemical and electronic properties1,2,3. Aggregates containing metal cation clusters ‘wrapped’ by lipophilic molecular anions have, for example, been shown4,5 to be kinetically stable and soluble in nonpolar liquids such as saturated hydrocarbons. The formation of supramolecular aggregates can even be exploited to generate aromatic hydrocarbons that carry four negative charges and crystallize in the form of organic poly(metal cation) clusters6,7 or helical polymers8. Here we report the anaerobic crystallization of an ionic organic aggregate—a contact ion septuple consisting of a fourfold negatively charged ‘tripledecker’ of three anthracene molecules bridged by four solvated potassium cations. Its electronic ground state is shown experimentally, using temperature-dependent electron paramagnetic resonance spectroscopy, to be a triplet. Although the spins in this biradical ionic solid are separated by a considerable distance, density functional theory calculations9 indicate that the triplet ground state is 84 kJ mol-1 more stable than the first excited singlet state. We expect that the successful crystallization of the ionic solid we report here, and that of a covalent organic compound with a triplet ground state10 at room temperature, will stimulate further attempts to develop new triplet-ground-state materials for practical use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lehn, J. M. Supramolecular Chemistry, Concepts and Perspectives (VCH, Weinheim, 1995).
Müller, A., Reuter, H. & Dillingers, S. Supramolecular inorganic chemistry: small guests in small and large hosts. Angew. Chem. Int. Edn Engl. 34, 2326–2361 (1995).
Müller, A., Shah, S. Q. N., Bögge, H. & Schmidtmann, M. Molecular growth from a Mo176 to a Mo248 cluster. Nature 397, 48–50 ( 1999).
Bock, H., Lehn, J. M., Pauls, J., Holl, S. & Krenzel, V. Sodium salts of the bipyridine dianion polymer[(bpy)2-{Na+(dme)}2]x cluster [(Na8O)6+Na+6(bpy)2-6(tmeda)6], and monomer [(bpy)2-{Na+(pmdta)}2]. Angew. Chem. Int. Edn. Engl. 38, 952–955 (1999).
Bock, H. et al. The lipophilically wrapped polyion aggregate {[Ba6Li 3O2]11+[-OC(CH3) 3]11 (OC4H8)3}, a face-sharing (octahedron + prismane) Ba6Li3O2 polyhedron in a hydrocarbon ellipsoid: preparation, single crystal structure analysis, and density functional calculations. Angew. Chem. Int. Edn. Engl. 34, 1353–1355 ( 1995).
Bock, H., Gharagozloo-Hubmann, K., Näther, C., Nagel, N. & Havlas, Z. [{Na+(thf) 2}4(rubrene)4-] crystallisation and structure determination of a contact ion quintuple for the first π-hydrocarbon-tetraanion. Angew. Chem. Int. Edn Engl. 35, 631– 633 (1996).
Sekiguchi, A., Matsuo, T. & Kabuto, C. Synthesis and characterisation of the tetralithium salt of an octasilyl-substituted trimethylencyclopentadien-tetraanion. Angew. Chem. Int. Edn Engl. 36, 2462– 2464 (1997).
Bock, H., Gharagozloo-Hubmann, K., Sievert, M. & Havlas, Z. Synthesis, crystal growth and structure determination of [(6,13′-Dipentacene⊝4)(K⊕DME)4]∞: a twin-strand polymer helix. Angew. Chem. Int. Edn Engl. (submitted).
Havlas, Z. & Bock, H. Bare molecular anions of the saturated hydrocarbons: density functional charge and spin distribution based on their single crystal structures. Collect. Czech. Chem. Commun. 63, 1245–1263 (1998).
Bock, H., John, A., Havlas, Z. & Bats, J. W. The triplet biradical tris(3,5-di(tert-butyl)4-oxophenylene)methane: crystal structure, spin and charge distribution. Angew. Chem. Int. Edn Engl. 32 , 416–418 (1993).
Bock, H., Näther, C., Ruppert, K. & Havlas, Z. Tetraphenylbutadiene disodium dimethoxyethene: solvent-shared and solvent-separated ion triples within a single crystal. J. Am. Chem. Soc. 114, 6907–6908 (1992).
Bock, H., Näther, C., Ruppert, K. & Havlas, Z. Competing Na⊕ solvation: ether-shared and ether-separated triple ions of perylene dianion. J. Am. Chem. Soc. 117, 3869–3870 (1995).
Bock, H. et al. Distorted molecules: perturbation design, preparation and structures. Angew. Chem. Int. Edn Engl. 31, 550– 581 (1992).
Bock, H., Näther, C., Havlas, Z., John, A. & Arad, C. Ether-solvated sodium ions in salts containing π-hydrocarbon anions: crystallisation, structures and semiempirical solvation energies. Angew. Chem. Int. Edn Engl. 33, 875–878 (1994).
Bock, H., Arad, C., Näther, C. & Havlas, Z. The structure of solvent-separated naphthalene and anthracene radical anions. J. Chem. Soc. Chem. Commune. 2393– 2394 (1995).
Rhine, W. E., Davis, J. & Stucky, G. Anthracene dianion dilithium bis(TMEDA). J. Am. Chem. Soc. 97, 2079–2085 (1975).
Stoll, S., Jeschke, G., Willer, M. & Schweiger, A. Nutation-frequency correlated EPR spectroscopy: the PEANUT experiment. J. Magn. Reson. 130, 86–96 ( 1998).
Acknowledgements
We thank T. Hauck for the first crystal growth, C. Näther and S. Holl for structure determination, V. Krenzel for graphics, and J. T. Töring & H. Käß for EPR measurements. This work was supported by the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie and the State of Hesse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bock, H., Gharagozloo-Hubmann, K., Sievert, M. et al. Single crystals of an ionic anthracene aggregate with a triplet ground state. Nature 404, 267–269 (2000). https://doi.org/10.1038/35005048
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35005048
This article is cited by
-
Redox-controlled potassium intercalation into two polyaromatic hydrocarbon solids
Nature Chemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.