Abstract
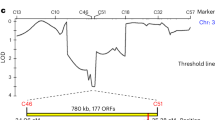
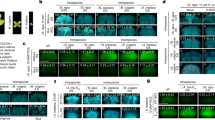
The self-incompatibility possessed by Brassica is an intraspecific reproductive barrier by which the stigma rejects self-pollen but accepts non-self-pollen for fertilization. The molecular/biochemical bases of recognition and rejection have been intensively studied. Self-incompatibility in Brassica is sporophytically controlled by the polymorphic S locus1. Two tightly linked polymorphic genes at the S locus, S receptor kinase gene (SRK) and S locus glycoprotein gene (SLG), are specifically expressed in the papillar cells of the stigma2,3,4, and analyses of self-compatible lines5,6,7 of Brassica have suggested that together they control stigma function in self-incompatibility interactions. Here we show, by transforming self-incompatible plants of Brassica rapa with an SRK28 and an SLG 28 transgene separately, that expression of SRK28 alone, but not SLG28 alone, conferred the ability to reject self (S28)-pollen on the transgenic plants. We also show that the ability of SRK28 to reject S28 pollen was enhanced by SLG28. We conclude that SRK alone determines S haplotype specificity of the stigma, and that SLG acts to promote a full manifestation of the self-incompatibility response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bateman, A. J. Self-incompatibility systems in angiosperms. III. Cruciferae. Heredity 9, 52–68 ( 1955).
Stein, J. C. et al. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc. Natl Acad. Sci. USA 88, 8816– 8820 (1991).
Takayama, S. et al. Sequences of S-glycoproteins, products of the Brassica campestris self-incompatibility locus. Nature 326 , 102–105 (1987).
Nasrallah, J. B., Kao, T.-h., Chen, C.-h., Goldberg, M. L. & Nasrallah, M. E. Amino-acid sequence of glycoproteins encoded by three alleles of the S-locus of Brassica oleracea. Nature 326, 617–619 ( 1987).
Goring, D. R., Glavin, T. L., Schafer, U. & Rothstein, S. J. An S receptor kinase gene in self-compatible Brassica napus has a 1-bp deletion. Plant Cell 5, 531– 539 (1993).
Nasrallah, J. B., Rundle, S. J. & Nasrallah, M. E. Genetic evidence for the requirement of the Brassica S-locus receptor kinase gene in the self-incompatibility response. Plant J. 5, 373–384 ( 1994).
Nasrallah, M. E., Kandasamy, M. K. & Nasrallah, J. B. A genetically defined trans-acting locus regulates S-locus function in Brassica. Plant J. 2 , 497–506 (1992).
Goring, D. R. & Rothstein, S. J. The S-locus receptor kinase gene in a self-incompatible Brassica napus line encodes a functional serine/threonine kinase. Plant Cell 4, 1273 –1281 (1992).
Nasrallah, J. B. & Nasrallah, M. E. Pollen–stigma signaling in the sporophytic self-incompatibility response. Plant Cell 5, 1325–1335 ( 1993).
Hinata, K., Watanabe, M., Toriyama, K. & Isogai, A. A review of recent studies on homomorphic self-incompatibility. Int. Rev. Cytol. 143, 257–296 (1993).
Suzuki, G. et al. Genomic organization of the S locus: Identification and characterization of genes in SLG/SRK region of S9 haplotype of Brassica campestris (syn. rapa). Genetics 153, 391–400 (1999).
Schopfer, C. R., Nasrallah, M. E. & Nasrallah, J. B. The male determinant of self-incompatibility in Brassica. Science 286, 1697– 1700 (1999).
Takasaki, T. et al. Introduction of SLG (S-locus glycoprotein) alters the phenotype of endogenous S-haplotype, but confers no new S-haplotype specificity in Brassica rapa L. Plant Mol. Biol. 40, 659–668 (1999).
Conner, J. A. et al. Transgene-induced silencing of S-locus genes and related genes in Brassica. Plant J. 11, 809 –823 (1997).
Stahl, R. J., Arnoldo, M., Glavin, T. L., Goring, D. R. & Rothstein, S. J. The self-incompatibility phenotype is altered by the transformation of a mutant S locus receptor kinase. Plant Cell 10, 209–218 (1998).
Hatakeyama, K., Takasaki, T., Watanabe, M. & Hinata, K. Molecular characterization of S locus genes, SLG and SRK , in a pollen-recessive self-incompatibility haplotype of Brassica rapa L. Genetics 149, 1587– 1597 (1998).
Hatakeyama, K., Watanabe, M. Takasaki, T., Ojima, K. & Hinata, K. Dominance relationships between S alleles in self-incompatible Brassica campestris L. Heredity 80, 241–247 ( 1998).
Watanabe, M. et al. A high degree of homology exists between the protein encoded by SLG and the S receptor domain encoded by SRK in self-incompatible Brassica campestris L. Plant Cell Physiol. 35, 1221–1229 (1994).
Suzuki, G., Watanabe, M., Toriyama, K., Isogai, A. & Hinata, K. Expression of SLG9 and SRK9 genes in transgenic tobacco. Plant Cell Physiol. 37, 866– 869 (1996).
Kish-Nishizawa, N. et al. Ultrastructure of papillar cells in Brassica campestris revealed by liquid helium rapid-freezing and substitute-fixation method. Plant Cell Physiol. 31, 1207– 1219 (1990).
Stone, S. L., Arnoldo, M. & Goring, D. R. A breakdown of Brassica self-incompatibility in ARC1 antisense transgenic plants. Science 286, 1729–1731 (1999).
Suzuki, G., Watanabe, M., Toriyama, K., Isogai, A. & Hinata, K. Molecular cloning of members of the S-multigene family in self-incompatible Brassica campestris. Plant Cell Physiol. 36, 1273–1280 (1995).
Jones, J. G. et al. Effective vectors for transformation, expression of heterologous, and assaying transposon excision in transgenic plant. Transgenic Res. 1, 285–297 ( 1992).
Takasaki, T. et al. Factors influencing Agrobacterium-mediated transformation of Brassica rapa L. Breed. Sci. 47, 127–134 (1997).
Kho, Y. O. & Bear, J. Observing pollen tubes by means of fluorescence. Euphytica 17, 298– 302 (1968).
Manolson, M. F., Oucllette, B. F., Filion, M. & Poole, R. J. cDNA sequence and homologies of the ‘57-Kda’ nucleotide-binding subunit of the vacuolar ATPase from Arabidopsis. J. Biol. Chem. 263, 17987–17994 ( 1988).
Nishio, T., Kusaba, M., Watanabe, M. & Hinata, K. Registration of S alleles in Brassica campestris L. by the restriction fragment sizes of SLGs. Theor. Appl. Genet. 92, 388–394 (1996).
Nishio, T., Kusaba, M., Sakamoto, K. & Ockendon, D. Polymorphism of the kinase domain of the S-locus receptor kinase gene (SRK) in Brassica oleracea L. Theor. Appl. Genet. 95, 335–342 (1997).
Brace, J., Ockendon, D. J. & King, G. J. Development of a method for identification of S alleles in Brassica oleracea based on digestion of PCR-amplified DNA with restriction endonucleases. Sex. Plant Reprod. 6, 133–138 (1993).
Shiba, H., Hinata, K., Suzuki, A. & Isogai, A. Breakdown of self-incompatibility in Brassica by the antisense RNA of the SLG gene. Proc. Jpn. Acad. B 71, 81–83 (1995).
Acknowledgements
We thank T.-h. Kao for his critical review and editing of the manuscript. This work was supported in part by Grants-in-Aid for Special research on Priority Areas from the Ministry of Education, Science, Culture and Sports, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takasaki, T., Hatakeyama, K., Suzuki, G. et al. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403, 913–916 (2000). https://doi.org/10.1038/35002628
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35002628
This article is cited by
-
Genome-wide identification and characterization of SRLK gene family reveal their roles in self-incompatibility of Erigeron breviscapus
BMC Genomics (2023)
-
Patterns of Diversity of the S and M Loci in Tunisian Apricots (Prunus armeniaca L.): Identification of Pollen-Part Mutations Conferring Self-Compatibility
Plant Molecular Biology Reporter (2023)
-
Self-incompatibility: a targeted, unexplored pre-fertilization barrier in flower crops of Asteraceae
Journal of Plant Research (2023)
-
MLPK function is not required for self-incompatibility in the S29 haplotype of Brassica rapa L.
Plant Reproduction (2023)
-
Ancestral self-compatibility facilitates the establishment of allopolyploids in Brassicaceae
Plant Reproduction (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.