Abstract
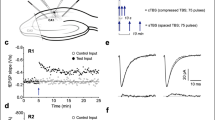
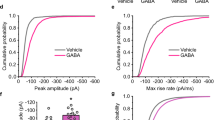
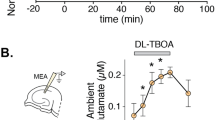
UNDERSTANDING the mechanisms involved in long-term potenti-ation (LTP) should provide insights into the cellular and molecular basis of learning and memory in vertebrates1. It has been established that in the CA1 region of the hippocampus the induction of LTP requires the transient activation of the N-methyl-D-aspartate (NMDA) receptor system2. During low-frequency transmission, significant activation of this system is prevented by γ-aminobutyric acid (GABA) mediated synaptic inhibition3,4 which hyperpolarizes neurons into a region where NMDA receptor-operated channels are substantially blocked by Mg2+ (refs. 5, 6). But during high-frequency transmission, mechanisms are evoked that provide sufficient depolarization of the postsynaptic membrane to reduce this block7 and thereby permit the induction of LTP. We now report that this critical depolarization is enabled because during high-frequency transmission GABA depresses its own release by an action on GABAB autoreceptors, which permits sufficient NMDA receptor activation for the induction of LTP. These findings demonstrate a role for GABAB receptors in synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bliss, T. V. P. & Lynch, M. A. Neurol Neurobiol. 35, 3–72 (1988).
Collingridge, G. L., Kehl, S. J. & McLennan, H. J. Physiol., Lond. 334, 33–46 (1983).
Dingledine, R., Hynes, M. A. & King, G. L., J. Physiol., Lond. 380, 175–189 (1986).
Collingridge, G. L., Herron, C. E. & Lester, R. A. J. J. Physiol., Lond. 399, 283–300 (1988).
Ascher, P. & Nowak, L. J. Physiol., Lond. 399, 247–266 (1988).
Mayer, M. L. & Westbrook, G. L. J. Physiol., Lond. 361, 65–90 (1985).
Collingridge, G. L., Herron, C. E. & Lester, R. A. J. J. Physiol., Lond. 399, 301–312 (1988).
Ben-Ari, Y., Krnjevic, K. & Reinhardt, W. Can. J. Physiol. Pharmacol. 57, 1462–1466 (1979).
McCarren, M. & Alger, B. E. J. Neurophysiol. 53, 557–571 (1985).
Harrison, N. L. J. Physiol., Lond. 422, 433–446 (1990).
Davies, C. H., Davies, S. N. & Collingridge, G. L. J. Physiol., Lond. 424, 513–531 (1990).
Olpe, H.-R. et al. Eur. J. Pharmac. 187, 27–38 (1990).
Larson, J. & Lynch, G. Brain Res. 441, 111–118 (1988).
Diamond, D. M., Dunwiddie, T. V. & Rose, G. M. J. Neurosci. 8, 4079–4088 (1988).
Coan, E. J. & Collingridge, G. L. Neurosci. Lett. 53, 21–26 (1985).
Lanthorn, T. H. & Cotman, C. W. Brain Res. 225, 171–178 (1981).
Randall, A. D., Schofield, J. G., Davies, C. H. & Collingridge, G. L. J. Physiol., Lond. 426, 51P (1990).
Dutar, P. & Nicoll, R. A. Nature 332, 156–158 (1988).
Dutar, P. & Nicoll, R. A. Neuron 1, 585–591 (1988).
Wigström, H. & Gustafsson, B. Nature 301, 603–604 (1983).
Mott, D. D., Lewis, D. V., Ferrari, C. M., Wilson, W. A. & Swartzwelder, H. S. Neurosci. Lett. 113, 222–226 (1990).
Olpe, H.-R. & Karlsson, G. Naunyn-Schmiedebergs Archs Pharmak. 342, 194–197 (1990).
Deisz, R. A. & Prince, D. A. J. Physiol., Lond. 412, 513–542 (1989).
Kauer, J. A., Malenka, R. C. & Nicoll, R. A. Neuron 1, 911–917 (1988).
Muller, D., Joly, M. & Lynch, G. Science 242, 1694–1697 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davies, C., Starkey, S., Pozza, M. et al. GABAB autoreceptors regulate the induction of LTP. Nature 349, 609–611 (1991). https://doi.org/10.1038/349609a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/349609a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.