Abstract
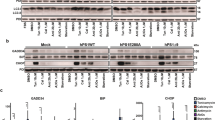
Point mutations in the presenilin-1 gene (PS1) are a major cause of familial Alzheimer's disease. They result in a selective increase in the production of the amyloidogenic peptide amyloid-β(1–42) by proteolytic processing of the amyloid precursor protein (APP)1,2,3,4. Here we investigate whether PS1 is also involved in normal APP processing in neuronal cultures derived from PS1-deficient mouse embryos. Cleavage by α- and β-secretase5 of the extracellular domain of APP was not affected by the absence of PS1, whereas cleavage by γ-secretase of the transmembrane domain of APP was prevented, causing carboxyl-terminal fragments of APP to accumulate and a fivefold drop in the production of amyloid peptide. Pulse-chase experiments indicated that PS1 deficiency specifically decreased the turnover of the membrane-associated fragments of APP. As in the regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor6, PS1 appears to facilitate a proteolytic activity that cleaves the integral membrane domain of APP. Our results indicate that mutations in PS1 that manifest clinically cause a gain of function and that inhibition of PS1 activity is a potential target for anti-amyloidogenic therapy in Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sherrington, R.et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
Scheuner, D.et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to Alzheimer's disease. Nat. Med. 2, 864–870 (1996).
Hardy, J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 20, 154–159 (1997).
Haass, C. Presenilins: genes for life and death. Neuron 18, 687–690 (1997).
Haass, C. & Selkoe, D. J. Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell 75, 1039–1042 (1993).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Wong, P. C.et al. Presenilin 1 is required for Notch 1 and Dll1 expression in the paraxial mesoderm. Nature 387, 288–292 (1997).
Shen, J.et al. Skeletal and CNS defects in presenilin-1 deficient mice. Cell 89, 629–639 (1997).
De Strooper, B.et al. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 14, 4932–4938 (1995).
Tienari, P.et al. Intracellular and secreted Alzheimer β-amyloid species are generated by distinct mechanisms in cultured hippocampal neurons. Proc. Natl Acad. Sci. USA 94, 4125–4130 (1997).
Simons, M.et al. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J. Neurosci. 16, 899–908 (1996).
Saftig, P.et al. Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J. Biol. Chem. 44, 27241–27244 (1996).
Haass, C.et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Chyung, A. S. C.et al. Novel β-secretase cleavage of amyloid precursor protein in the endoplasmic reticulum/intermediate compartment of NT2N cells. J. Cell Biol. 138, 671–680 (1997).
Citron, M.et al. Evidence that the 42- and 40-amino acid forms of amyloid β protein are generated from the β-amyloid precursor protein by different protease activities. Proc. Natl Acad. Sci. USA 93, 13170–13175 (1996).
Klafki, H. W.et al. The carboxy-terminal termini of β-amyloid peptides 1–40 and 1–42 are generated by distinct γ-secretase activities. J. Biol. Chem. 45, 28655–28659 (1996).
Hartmann, T.et al. Distinct sites of intracellular production for Alzheimer's disease Aβ40/42-amyloid peptides. Nature Med. 3, 1016–1020 (1997).
Weidemann, A.et al. Formation of stable complexes between two Alzheimer's disease gene products: presenilin 2 and β-amyloid precursor protein. Nature Med. 3, 328–332 (1997).
Xia, W.et al. Interaction between amyloid precursor protein and presenilins in mammalian cells: implications for the pathogenesis of Alzheimer's disease. Proc. Natl Acad. Sci. USA 94, 8208–8213 (1997).
Doan, A.et al. Protein topology of presenilin 1. Neuron 17, 1023–1030 (1996).
Li, X. & Greenwald, I. Membrane topology of the C. elegans SEL-12 presenilin. Neuron 17, 1017–1021 (1996).
Lehmann, S., Chiesa, R. & Harris, D. A. Evidence for a six-transmembrane domain structure of presenilin-1. J. Biol. Chem. 272, 12047–12051 (1997).
De Strooper, B.et al. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer's disease-associated presenilins. J. Biol. Chem. 272, 3590–3598 (1997).
Levitan, D.et al. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 93, 14940–14944 (1996).
Baumeister, R.et al. Human presenilin-1, but not familial Alzheimer's disease (FAD) mutants, facilitate Caenorrhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct. 1, 149–159 (1997).
Citron, M.et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Med. 3, 67–72 (1997).
Vanderstichele, H.et al. in Progress in Alzheimer's and Parkinson's Diseases (eds Fisher, A., Yoshida, M. & Hanin, I.) (Plenum, New York, in the press).
Barelli, H. Characterization of new polyclonal antibodies specific for 40- and 42-amino-acids-long amyloid β peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol. Med. 3, 695–707 (1997).
De Strooper, B.et al. Basolateral secretion of amyloid precursor protein in MDCK cells is disturbed by alterations of intracellular pH and by introducing a mutation associated with familial Alzheimer's disease. J. Biol. Chem. 270, 4058–4065 (1995).
Acknowledgements
This work was supported by the FWO-Vlaanderen, the Flemish Action Program for Biotechnology (VLAB), the Flemish Institute for Biotechnology (VIB), the K.U.Leuven, the Human Frontier of Science Program (HFSP), the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie. We thank B. Greenberg for antibodies anti-APP1-612 and anti-APPKM595/596, D. Selkoe for R1736 and R1282, and F. Checler for FCA3340, C. Dotti, B. Nelissen and E.Vanmechelen for advice; and S. Tiereliers for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
De Strooper, B., Saftig, P., Craessaerts, K. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998). https://doi.org/10.1038/34910
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/34910
This article is cited by
-
Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future
Signal Transduction and Targeted Therapy (2023)
-
A proteomics analysis of 5xFAD mouse brain regions reveals the lysosome-associated protein Arl8b as a candidate biomarker for Alzheimer’s disease
Genome Medicine (2023)
-
The role of Smo-Shh/Gli signaling activation in the prevention of neurological and ageing disorders
Biogerontology (2023)
-
Turning the tide on Alzheimer’s disease: modulation of γ-secretase
Cell & Bioscience (2022)
-
Molecular basis for isoform-selective inhibition of presenilin-1 by MRK-560
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.