Abstract
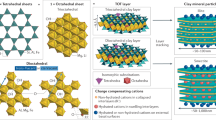
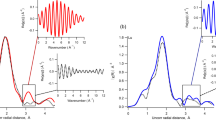
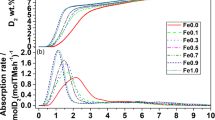
METALS dissolved in natural waters often become sorbed onto oxide or clay minerals, so that prediction of their chemical behaviour and transport properties requires knowledge of the structure and bonding of metal species at the solid/water interface1. For many sorption systems, X-ray absorption spectroscopy (XAS) can be used to determine the identity and number of nearest-neighbour atoms and interatomic distances in aqueous complexes on solid surfaces, and thus to identify the dominant type of surface complex and the partitioning mechanism2. Here we describe an XAS study of divalent cobalt (Co(II)) complexes sorbed on three different solids, γ-Al2O3, rutile (TiO2) and kaolinite (Al2Si2O5(OH)4). We find direct evidence for the presence of multinuclear sorption complexes at surface coverages below one monolayer of Co(II) atoms. Our spectroscopic data reveal distinct differences in the number of coordinating atoms and interatomic distances in the surface complexes formed on each of the solids at the same sorption density. These results suggest that different oxide and clay surfaces influence the structure and properties of aqueous surface complexes, and therefore must be accounted for in models of metal-ion sorption.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Parks, G. A. Chem. Aust. 49, 389–395 (1981).
Sposito, G. Chimia 43, 169–176 (1989).
James, R. O. & Healy, T. W. J. Colloid Interface Sci. 40, 53–64 (1972).
Matijevic, E. J. Colloid Interface Sci. 43, 217–245 (1983).
Tripathi, V. S. thesis, Stanford Univ. (1983).
Farley, K. J., Dzombak, D. A. & Morel, F. M. M. J. Colloid Interface Sci. 106, 226–242 (1985).
Tewari, P. H. & Lee, W. J. Colloid Interface Sci. 52, 77–88 (1975).
Bleam, D. F. & McBride, M. B. J. Colloid Interface Sci. 103, 124–132 (1985).
Motschi, H. in Aquatic Surface Chemistry (ed. Stumm, W.) 111–125 (Wiley, New York, 1987).
Sposito, G. Surface Chemistry of Soils (Oxford University Press, New York, 1984).
Davis, J. A., James, R. O. & Leckie, J. O. J. Colloid Interface Sci. 63, 480–499 (1978).
Brown, G. E. Jr & Parks, G. A. Rev. Geophys. 27, 519–533 (1989).
Brown, G. E. Jr, Parks, G. A. & Chisholm-Brause, C. J. Chimia 43, 248–256 (1989).
Chisholm-Brause, C. J. et al. Geochim. cosmochim. Acta 54, 1897–1909 (1990).
Hayes, K. F. et al. Science 238, 783–786 (1987).
Teo, B-K. & Lee, P. A. J. Am. chem. Soc. 101, 2815–2832 (1979).
Stern, E. A. in X-ray Absorption (eds Kroningsberger, D. C. & Prins, R.) 3–51 (Wiley, New York, 1988).
Brown, G. E. Jr, Calas, G., Waychunas, G. A. & Petiau, J. in Spectroscopic Methods in Mineralogy and Geology (ed. Hawthorne, F. C.) 431–512 (Miner. Soc. Am., Chelsea, Michigan, 1988).
Heald, S. M. in X-ray Absorption (eds Kroningsberger, D. C. & Prins, R.) 87–118 (Wiley, New York, 1988).
Cramer, S. P. & Hodgson, K. O. Prog. inorg. Chem. 25, 1–39 (1979).
Sayers, D. C. & Bunker, B. A. in X-ray Absorption (eds Kroningsberger, D. C. & Prins, R.) 211–253 (Wiley, New York, 1988).
Waychunas, G. A., Brown, G. E. Jr & Apted, M. J. Phys. Chem. Miner 13, 31–47 (1986).
Cramer, S. P., Hodgson, K. O., Stiefel, E. I. & Newton, W. E. J. Am. chem. Soc. 100, 1144–1150 (1983).
Co, M. S., Hendrickson, W. A., Hodgson, K. O. & Doniach, S. J. Am. chem. Soc. 105, 2748–2761 (1978).
O'Day, P. A., Brown, G. E. Jr & Parks, G. A. in Proc. 6th Int. Conf. on X-ray Absorption Fine Structure (ed. S. S. Hasnain) (Ellis-Horwood Publishers, in the press).
Hachiya, K., Sasaki, M., Saruta, Y., Mikami, N. & Yasunaga, T. J. phys. Chem. 88, 23–27 (1984).
Baes, C. F. Jr & Mesmer, R. E. The Hydrolysis of Cations (Wiley, New York, 1986).
Lotmar, V. W. & Feitknecht, W. Z. Kristallogr. A93, 368–378 (1936).
May, H. M., Kinniburgh, D. G., Helmke, P. A. & Jackson, M. L. Geochim. cosmochim. Acta 50, 1667–1677 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chisholm-Brause, C., O'Day, P., Brown, G. et al. Evidence for multinuclear metal-ion complexes at solid/water interfaces from X-ray absorption spectroscopy. Nature 348, 528–531 (1990). https://doi.org/10.1038/348528a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/348528a0
This article is cited by
-
Adsorptive removal of strontium ions from aqueous solution by graphene oxide
Environmental Science and Pollution Research (2019)
-
Interaction of radionuclides with natural and manmade materials using XAFS technique
Science China Chemistry (2017)
-
NMR imaging study of various stages of a supported catalyst preparation process: getting the qualitative and quantitative information
Applied Magnetic Resonance (2014)
-
Applications of synchrotron-based X-ray techniques in environmental science
Science China Chemistry (2010)
-
Comparative study on sorption of radiocobalt to montmorillonite and its Al-pillared and cross-linked samples: Effect of pH, ionic strength and fulvic acid
Journal of Radioanalytical and Nuclear Chemistry (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.