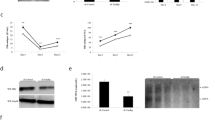
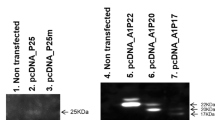
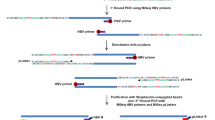
Abstract
HEPATITIS B virus (HBV) is regarded as the main aetiologic factor in the development of human hepatocellular carcinoma (HCC), one of the most frequent fatal malignancies worldwide1,2. Detection of integrated HBV sequences in the cellular DNA of almost all HCCs studied2–17, and the recent finding that the integrated HBV open reading frame (orf) X encodes a trans-activating activity18, supports the notion that integrated HBV DNA could contribute to liver carcinogenesis by activation of cellular genes in trans18,20. But not all HCCs seem to harbour a functional orf X2–17. We report here that 3′-truncated preS2/Ssequences in integrated HBV DNA of liver cell carcinomas encode a so far unidentified transcriptional trans-activation activity. This activity is also produced by an artificially 3′-truncated preS2/S gene of the wild-type HBV genome. Besides the simian virus 40 promoter of the reporter plasmid pSV2CAT, the promoter of the human c-myc oncogene can also be activated. These results, taken together with the fact that preS/S is the only HBV gene found to be integrated in almost every HBV-related HCC analysed so far2–17, indicate that trans-activation by integrated preS2/S sequences is a possible mechanism for HBV-associated oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Beasley, R. P. & Hwang, L. Y. in Viral Hepatitis and Liver Disease (eds Vyas, G. N., Dienstag, J. L. & Hoofnagle, J. H.) 209–214 (Grune & Stratton, New York, 1984).
Ganem, D. & Varmus, H. E. A. Rev. Biochem. 56, 651–693 (1987).
Bréchot, C., Pourcel, C., Louise, A., Rain, B. & Tiollais, P. Nature 286, 533–535 (1980).
Dejean, A., Bougueleret, L., Grzeschik, K.-H. & Tiollais, P. Nature 322, 70–72 (1986).
Edman, J. C., Gray, P., Valenzuela, P., Rail, L. B. & Rutter, W. J. Nature 286, 535–538 (1980).
Koshy, R. et al. Cell 34, 215–223 (1983).
Yaginuma, K., Kobayashi, M., Yoshida, E. & Koike, K. Proc. natn. Acad. Sci. U.S.A. 82, 4458–4462 (1985).
Berger, I. & Shaul, Y. J. Virol. 61, 1180–1186 (1987).
Choo, K.-B. et al. Virology 154, 405–408 (1986).
Fowler, M. J., Thomas, H. C. & Monjardino, J. J. gen. Virol. 67, 771–775 (1986).
Imai, M. et al. J. Virol. 61, 3555–3560 (1987).
Koike, K. et al. Nucleic Acids Res. 11, 5391–5402 (1983).
Nagaya, T. et al. Genes Dev. 1, 773–782 (1987).
Ogston, C. W., Jonak, G. J., Rogler, C. E., Astrin, S. M. & Summers, J. Cell 29, 385–394 (1982).
Shaul, Y., Garcia, P. D., Schonberg, S. & Rutter, W. J. J. Virol. 59, 731–734 (1986).
Tur-Kaspa, R., Burk, R. D., Lieberman, H. H. & Shafritz, D. A. J. Hepat. 3, S25–S33 (1986).
Ziemer, M., Garcia, P., Shaul, Y. & Rutter, W. J. J. Virol. 53, 885–892 (1985).
Wollersheim, M., Debelka, U. & Hofschneider, P.-H. Oncogene 3, 545–554 (1988).
Twu, J.-S. & Schloemer, R. H. J. Virol. 61, 3448–3453 (1987).
Zahm, P., Hofschneider, P. H. & Koshy, R. Oncogene 3, 169–177 (1988).
Huh, N. & Utakoji, T. Gann 72, 178–179 (1981).
Gorman, C. M., Moffat, L. F. & Howard, B. H. Molec. cell. Biol. 2, 1044–1051 (1982).
Kaneko, S. & Miller, R. J. Virol. 11, 3979–3984 (1988).
Haslinger, A. & Karin, M. Proc. natn. Acad. Sci. U.S.A. 82, 8572–8576 (1985).
Cattaneo, R., Will, H., Hernandez, N. & Schaller, H. Nature 305, 336–338 (1983).
Standring, O. N., Rutter, W. J., Varmus, H. E. & Ganem, D. J. Virol. 50, 563–571 (1984).
Will, H. et al. Proc. natn. Acad. Sci. U.S.A. 82, 891–895 (1985).
Seto, E., Yen, T. B., Peterlin, B. M. & Ou, J.-H. Proc. natn. Acad. Sci. U.S.A. 85, 8286–8290 (1988).
Lipp, M., Schilling, R., Wiest, S., Laux, G. & Bornkamm, G. W. Molec. cell. Biol. 7, 1393–1400 (1987).
Budkowska, A., Dubreuil, P., Riottot, M.-M., Briantais, M.-J. & Pillot, J. J. immunol. Meth. 97, 77–85 (1987).
Heermann, K.-H., Waldeck, F. & Gerlich, W. H. in Viral Hepatitis and Liver Disease (ed. Zuckermann, A. J.) 697–700 (Liss, New York, 1988).
Marquardt, O., Heermann, K.-H., Seifer, M. & Gerlich, W. H. Archs Virol. 96, 249–256 (1987).
Graham, F. L. & van der Eb, A. J. Virology 52, 456–467 (1973).
Angel, P. et al. Cell 49, 729–739 (1987).
Chirgwin, J. M., Przybyla, A. E., MacDonald, R. J. & Rutter, W. J. Biochemistry 18, 5294–5299 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kekule, A., Lauer, U., Meyer, M. et al. The preS2/S region of integrated hepatitis B virus DNA encodes a transcriptional transactivator. Nature 343, 457–461 (1990). https://doi.org/10.1038/343457a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/343457a0
This article is cited by
-
Hepatitis B und C: Mechanismen der virusinduzierten Leberpathogenese und Tumorentstehung
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2022)
-
Long-read sequencing reveals the structural complexity of genomic integration of HBV DNA in hepatocellular carcinoma
npj Genomic Medicine (2021)
-
Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma
Nature Communications (2016)
-
Genetic and epigenetic alterations in hepatitis B virus-associated hepatocellular carcinoma
Virologica Sinica (2015)
-
Medical Virology of Hepatitis B: how it began and where we are now
Virology Journal (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.