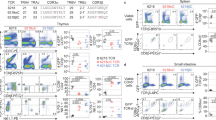
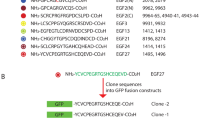
Abstract
IMMUNE responses to proteins necessarily involve the recognition by T lymphocytes of a peptide or peptides derived from a protein complexed with a major histocompatibility antigen. The T-cell response of BALB/c mice to the bacteriophage Λ cI represser protein (residues 1-102) is directed predominantly towards the epitope contained within a single peptide encompassing residues 12–26 (refs 1, 2). Similar phenomena of immunodominance of a particular peptide have also been observed in other protein systems3–6. The mechanisms that have been suggested to account for the focusing of the T-cell response7are partial deletion in the T-cell repertoire, biased antigen processing, and competition for binding to the presenting molecule, the major histocompatibility complex encoded class II transplantation antigen. In a model system with a polypeptide containing two synthetically linked immunologically active epitopes, we now demonstrate the existence of a hierarchy between these epitopes, so that the immune response elicited is directed mainly towards the more immunogenic epitope, whereas the less immunogenic epitope elicits little or no T-cell reactivity. In addition, the same hierarchy of dominance is also apparent when the polypeptide is used to induce tolerance in the periphery in adult mice. The chimaeric peptide can induce tolerance only towards the more immunogenic epitope. These experiments indicate that the rules governing antigen processing and presentation that result in T-cell activation are apparently the same as the rules that govern the processes resulting in the induction of tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Guillet, J. G. et al. Science 235, 865–870 (1987).
Roy, S., Scherer, M. T., Briner, T. J., Smith, J. A. & Gefter, M. L. Science 244, 572–575 (1989).
Allen, P. M., Matsueda, G. R., Haber, E. & Unanue, E. R. J. Immun. 135, 368–373 (1985).
Kuisaki, S., Atassi, H. & Atassi, M. Eur. J. Immun. 16, 236–240 (1986).
Townsend, A., Goth, F. & Davey, J. Cell 42, 457–462 (1985).
Shimonkewitz, R., Kappler, J., Marrack, P. & Grey, H. M. J. Immun. 133, 2067–2074 (1984).
Schaeffer, E. B. et al. Proc. natn. Acad. Sci. U.S.A. 86, 4649–4653 (1989).
Sette, A. et al. Nature 328, 395–399 (1987).
Shastri, N., Miller, A. & Sercarz, E. E. J. Immun. 136, 371–376 (1986).
Guillet, J. G., Lai, M. Z., Briner, T. J., Smith, J. A. & Gefter, M. L. Nature 324, 260–262 (1986).
Adorini, L. et al. Nature 334, 623–625 (1988).
Gammon, G. et al. Nature 319, 413–415 (1986).
Quill, H. & Schwartz, R. H. J. Immun. 138, 3704–3712 (1987).
Lamb, J. R. et al. J. exp. Med. 157, 1434–1447 (1983).
Markmann, J. et al. Nature 336, 476–479 (1988).
Lai, M. Z. et al. J. Immun. 139, 3973–3980 (1987).
Sauer, R. T. & Andregg, R. Biochemistry 17, 1092–1100 (1978).
Merrifield, R. B. J. Am. chem. Soc. 85, 2149–2154 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ria, F., Chan, B., Scherer, M. et al. Immunological activity of covalently linked T-cell epitopes. Nature 343, 381–383 (1990). https://doi.org/10.1038/343381a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/343381a0
This article is cited by
-
MHC-guided processing: binding of large antigen fragments
Nature Reviews Immunology (2003)
-
Signaling through OX40 (CD134) breaks peripheral T-cell tolerance
Nature Medicine (2001)
-
Antigen persistence and time of T-cell tolerization determine the efficacy of tolerization protocols for prevention of skin graft rejection
Nature Medicine (1998)
-
Synthetic peptides in biochemical research
Molecular Biotechnology (1995)
-
The immune system victorious: Selective preservation of self
Immunologic Research (1993)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.