Abstract
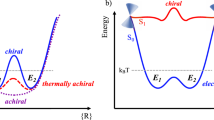
TRANSFER of chirality and multiplication of chiral molecules are key processes in the chemical and biochemical transformations of natural and synthetic compounds. For entropic reasons, structural immobilization in enzymatic and catalytic processes and the use of low temperatures should generally enhance the optical yield (preference for one enantiomer) in chiral reactions. Studies of the temperature dependence of optical yield in chemical and biochemical processes have, however, been sporadic1–10. Here we report a systematic study of the temperature dependence of the photosensitized enantiodifferentiating isomerization of a simple alkene. We find that above a critical temperature, characteristic of the photosensitizer used, the optical yield increases with increasing temperature, apparently conflicting with the widely accepted view that lower temperatures favour a higher optical yield. Thus both enantiomers may be produced with high efficiency using a single chiral source. This implies that transfer and multiplication of chirality in natural systems, an important aspect of the development of prebiotic organic molecules, may be effected rather more simply than has hitherto been supposed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Morrison, J. D. & Mosher, H. S. Asymmetric Organic Reactions (Prentice-Hall, Englewood Cliffs, 1971).
Izumi, Y. & Tai, A. Stereo-differentiating Reactions (Kodansha-Academic, Tokyo, 1977).
Retey, J. & Robinson, J. A. Stereospecificity in Organic Chemistry and Enzymology (Verlag Chemie, Weinheim, FRG, 1982).
Morrison, J. D. (ed.) Asymmetric Synthesis Vols 1–5 (Academic, New York, 1983–1984).
Coppola, G. M. & Schuster, H. F. (eds) Asymmetic Synthesis (Wiley-lnterscience, New York, 1987).
Harada, K. & Yoshida, T. JCS, chem. Commun. 1071 (1970); J. org. Chem. 37, 4366–4370 (1972).
Harada, K. & Kataoka, Y. Chem. Lett. 791–794 (1978).
Ojima, J., Kogure, T. & Toda, N. J. org. Chem. 45, 4728–4739 (1980).
Sinou, D. Tetrahedron Lett. 22, 2987–2990 (1981).
Landis, C. R. & Halpern, J. J. Am. chem. Soc. 109, 1746–1754 (1987).
Rau, H. Chem. Rev. 83, 535–547 (1983).
Hammond, G. S. & Cole, R. S. J. Am. chem. Soc. 87, 3256–3257 (1965).
Ouannes, C., Beugelmans, R. & Roussi, G. J. Am. chem. Soc. 95, 8472–8474 (1972).
Balavoine, G., Juge, S. & Kagan, H. B. Tetrahedron Lett. 4159–4162 (1973).
Inoue, Y., Kunitomi, Y., Takamuku, S. & Sakurai, H. JCS, chem. Commun. 1024–1025 (1978).
Inoue, Y., Takamuku, S., Kunitomi, Y. & Sakurai, H. JCS, Perkin Trans. II, 1672–1677 (1980).
Goto, S., Takamuku, S., Sakurai, H., Inoue, Y. & Hakushi, T. JCS, Perkin Trans. II, 1678–1681 (1980).
Cope, A. C., Ganellin, C. R., Johnson, H. W. Jr, Van Auken, T. V. & Winkler, H. J. S. J. Am. chem. Soc. 85, 3276–3279 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inoue, Y., Yokoyama, T., Yamasaki, N. et al. An optical yield that increases with temperature in a photochemically induced enantiomeric isomerization. Nature 341, 225–226 (1989). https://doi.org/10.1038/341225a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/341225a0
This article is cited by
-
Catalytic deracemization of chiral allenes by sensitized excitation with visible light
Nature (2018)
-
Dynamic control of chirality in phosphine ligands for enantioselective catalysis
Nature Communications (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.