Abstract
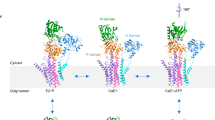
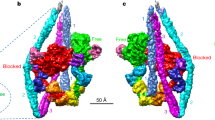
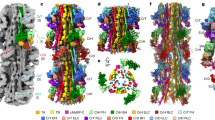
The calcium pump from sarcoplasmic reticulum (Ca2+-ATPase) is typical of the large family of P-type cation pumps. These couple ATP hydrolysis with cation transport, generating cation gradients across membranes. Ca2+-ATPase specifically maintains the low cytoplasmic calcium concentration of resting muscle by pumping calcium into the sarcoplasmic reticulum; subsequent release is used to initiate contraction. No high-resolution structure of a P-type pump has yet been determined, although a 14-Å structure ofCa2+-ATPase, obtained by electron microscopy of frozen-hydrated, tubular crystals1, showed a large cytoplasmic head connected to the transmembrane domain by a narrow stalk. We have now improved the resolution to 8 Å and can discern ten transmembrane α-helices, four of which continue into the stalk. On the basis of constraints from transmembrane topology, site-directed mutagenesis and disulphide crosslinking, we have made tentative assignments for these α-helices within the amino-acid sequence. A distinct cavity leads to the putative calcium-binding site, providing a plausible path for calcium release to the lumen of the sarcoplasmic reticulum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Toyoshima, C., Sasabe, H. & Stokes, D. L. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature 362, 469–471 (1993).
Dux, L. & Martonosi, A. Two-dimensional arrays of proteins in sarcoplasmic reticulum and purified Ca2+-ATPase vesicles treated with vanadate. J. Biol. Chem. 258, 2599–2603 (1983).
Taylor, K. A., Dux, L. & Martonosi, A. Three-dimensional reconstruction of negatively stained crystals of the Ca++-ATPase from muscle sarcoplasmic reticulum. J. Mol. Biol. 187, 417–427 (1986).
Stokes, D. L. & Lacapere, J.-J. Conformation of Ca2+-ATPase in two crystal forms: Effects of Ca2+, thapsigargin, AMP-PCP, and Cr-ATP on crystallization. J. Biol. Chem. 269, 11606–11613 (1994).
Hua, S., Malak, H., Lakowicz, J. R. & Inesi, G. Synthesis and interaction of fluorescent thapsigargin derivatives with the carcoplasmic reticulum ATPase membrane-bound region. Biochemistry 34, 5137–5142 (1995).
Sagara, Y., Wade, J. B. & Inesi, G. Aconformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J. Biol. Chem. 267, 1286–1292 (1992).
MacLennan, D. H., Brandl, C. J., Korczak, B. & Green, N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 316, 696–700 (1985).
Moller, J. V., Juul, B. & le Maire, M. Structural organization, ion transport, and energy transduction of ATPases. Biochim. Biophys. Acta 1286, 1–51 (1996).
MacLennan, D. H., Rice, W. J. & Green, N. M. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+ ATPases. J. Biol. Chem. 272, 28815–28818 (1997).
Gadsby, D. C., Rakowski, R. F. & De Weer, P. Extracellular access to the Na, K pump: Pathway similar to ion channel. Science 260, 100–103 (1993).
Hilgemann, D. W. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science 263, 1429–1432 (1994).
Stokes, D. L., Taylor, W. R. & Green, N. M. Structure, transmembrane topology and helix packing of P-type ion pumps. FEBS Lett. 346, 32–38 (1994).
Clarke, D. M., Loo, T. W., Inesi, G. & MacLennan, D. H. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature 339, 476–478 (1989).
Andersen, J. P. Dissection of the functional domains of the sarcoplasmic reticulum Ca2+-ATPase by site-directed mutagenesis. Biosci. Rep. 15, 243–261 (1995).
Rice, W. J. & MacLennan, D. H. Scanning mutagenesis reveals a similar pattern of mutation sensitivity in transmembrane sequences M4, M5, and M6, but not in M8, of the Ca2+-ATPase of sarcoplasmic reticulum (SERCA1a). J. Biol. Chem. 271, 31412–31419 (1996).
Chen, L. et al. Short and long range functions of amino acids in the transmembrane region of the sarcoplasmic reticulum ATPase: a mutational study. J. Biol. Chem. 271, 10745–10752 (1996).
Rice, W. J. & MacLennan, D. H. Site-directed disulfide mapping of helices M4 and M6 in the Ca2+ binding domain of SERCA1a, the Ca2+ATPase of fast-twitch skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 272, 31412–31419 (1997).
Baldwin, J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 12, 1693–1703 (1993).
Green, N. M. in The Sodium Pump: Structure, Mechanisms, Hormonal Control and its Role in Disease (eds Bamberg, E. & Schoner, W.) 110–119 (Steinkopff, Darmstadt, 1994).
Matthews, I., Sharma, R. P., Lee, A. G. & East, J. M. Transmembranous organization of (Ca2+-Mg2+)-ATPase from sarcoplasmic reticulum: evidence for lumenal location of residues 877–888. J. Biol. Chem. 265, 18737–18740 (1990).
Clarke, D. M., Loo, T. W. & MacLennan, D. H. The epitope for monoclonal antibody A20 (amino acids 870–890) is located on the luminal surface of the Ca2+-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 265, 17405–17408 (1990).
Beroukhim, R. & Unwin, N. Distortion correction of tubular crystals: Improvements in the acetylcholine receptor structure. Ultramicroscopy 70, 57–81 (1997).
Tani, K., Sasabe, H. & Toyoshima, C. Aset of computer programs for determining defocus and astigmatism in electron images. Ultramicroscopy 65, 31–44 (1997).
Toyoshima, C., Yonekura, K. & Sasabe, H. Contrast transfer for frozen-hydrated specimens: II. Amplitude contrast at very low frequencies. Ultramicroscopy 48, 165–176 (1993).
Green, N. M. ATP-driven cation pumps: alignment of sequences. Biochem. Soc. Trans. 17, 970–972 (1989).
Guerini, D., Foletti, D., Vellani, F. & Carofoli, E. Mutation of conserved residues in transmembrane domains 4, 6, and 8 causes loss of Ca2+ transport by the plasma membrane Ca2+ pump. Biochemistry 35, 3290–3296 (1996).
Acknowledgements
We thank R. Beroukhim and N. Unwin for the use of their programs for helical image analysis, and G. Inesi for the dansyl thapsigargin. This work was partly supported by the NIH and the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, P., Toyoshima, C., Yonekura, K. et al. Structure of the calcium pump from sarcoplasmic reticulum at 8-Å resolution. Nature 392, 835–839 (1998). https://doi.org/10.1038/33959
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/33959
This article is cited by
-
Targeting oncogenic Notch signaling with SERCA inhibitors
Journal of Hematology & Oncology (2021)
-
SERCA2a ameliorates cardiomyocyte T-tubule remodeling via the calpain/JPH2 pathway to improve cardiac function in myocardial ischemia/reperfusion mice
Scientific Reports (2021)
-
B-type natriuretic peptide and its role in altering Ca2+-regulatory proteins in heart failure—mechanistic insights
Heart Failure Reviews (2020)
-
The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain
Cellular and Molecular Neurobiology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.