Abstract
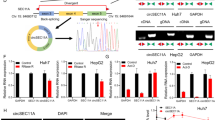
Due to limited treatment options the prognosis of patients with advanced hepatocellular cancer (HCC) has remained poor. To investigate an alternative therapeutic approach, we examined the feasibility of radioiodine therapy of HCC following human sodium iodide symporter (NIS) gene transfer using a mouse α-fetoprotein (AFP) promoter construct to target NIS expression to HCC cells. For this purpose, the murine Hepa 1–6 and the human HepG2 hepatoma cell lines were stably transfected with NIS cDNA under the control of the tumor-specific AFP promoter. The stably transfected Hepa 1–6 cell line showed a 10-fold increase in iodide accumulation, while HepG2 cells accumulated 125I approximately 60-fold. Tumor-specific NIS expression was confirmed on mRNA level by northern blot analysis, and on protein level by immunostaining, that revealed primarily membrane-associated NIS-specific immunoreactivity. In an in vitro clonogenic assay up to 78% of NIS-transfected Hepa 1–6 and 93% of HepG2 cells were killed by 131I exposure, while up to 96% of control cells survived. In vivo NIS-transfected HepG2 xenografts accumulated 15% of the total 123I administered per gram tumor with a biological half-life of 8.38 h, resulting in a tumor absorbed dose of 171 mGy MBq−1 131I. After administration of a therapeutic 131I dose (55.5 MBq) tumor growth of NIS expressing HepG2 xenografts was significantly inhibited. In conclusion, tumor-specific iodide accumulation was induced in HCC cells by AFP promoter-directed NIS expression in vitro and in vivo, which was sufficiently high to allow a therapeutic effect of 131I. This study demonstrates the potential of tumor-specific NIS gene therapy as an innovative treatment strategy for HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P . Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94: 153–156.
Bruix J, Sherman M . Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236.
Sangro B, Mazzollini G, Prieto J . Future therapies for hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2005; 17: 515–521.
Mazzaferri EL . 1996 Carcinoma of follicular epithelium: radioiodine and other treatments and outcomes. In: Braverman LE, Utiger RD (eds). The Thyroid: A Fundamental and Clinical Text, 7th edn. Lippincott, Raven: Philadelphia, pp 922–945.
Dai G, Levy O, Carrasco N . Cloning and characterization of the thyroid iodide transporter. Nature 1996; 379: 458–460.
Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL et al. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun 1996; 226: 339–345.
Spitzweg C, Morris JC . The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol 2002; 57: 559–574.
Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocrine Rev 2003; 24: 48–77.
Smanik PA, Ryu K-Y, Theil KS, Mazzaferri EL, Jhiang SM . Expression, exon–intron organization, and chromosome mapping of the human sodium iodide symporter. Endocrinology 1997; 138: 3555–3558.
Spitzweg C, Harrington KJ, Pinke LA, Vile RG, Morris JC . The sodium iodide symporter and its potential in cancer therapy. J Clin Endocrinol Metab 2001; 86: 3327–3335.
Hart IR . Tissue specific promoters in targeting systemically delivered gene therapy. Semin Oncol 1996; 23: 154–158.
Spitzweg C, Zhang S, Bergert ER, Castro MR, McIver BD, Heufelder AE et al. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res 1999; 59: 2136–2141.
Spitzweg C, O'Connor MK, Bergert ER, Tindall DJ, Young CYF, Morris JC . Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res 2000; 60: 6526–6530.
Kakinuma H, Bergert ER, Spitzweg C, Cheville JC, Lieber MM, Morris JC . Probasin promoter (ARR2PB)-driven, prostate-specific expression of h-NIS for targeted radioiodine therapy of prostate cancer. Cancer Res 2003; 63: 7840–7844.
Dingli D, Diaz RM, Bergert ER, O'Connor MK, Morris JC, Russell SJ . Genetically targeted radiotherapy for multiple myeloma. Blood 2003; 102: 489–496.
Dingli D, Russell SJ, Morris JC . In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem 2003; 90: 1079–1086.
Spitzweg C, Dietz AB, O'Connor MK, Bergert ER, Tindall DJ, Young CYF et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Therapy 2001; 8: 1524–1531.
Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodidie symporter gene transfer. Gene Therapy 2005; 12: 272–280.
Cengic N, Baker CH, Schutz M, Göke B, Morris JC, Spitzweg C . A novel therapeutic strategy for medullary thyroid cancer based on radioiodine therapy following tissue-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab 2005; 90: 4457–4464.
Spitzweg C, Baker CH, Bergert ER, O'Connor MK, Morris JC . Image-guided radioiodide therapy of medullary thyroid cancer following carcinoembryonic antigen (CEA)-promoter-targeted sodium iodide symporter (NIS) gene expression. Hum Gene Ther 2007; 18: 916–924.
Gorin MB, Tilghman SM . Structure of the α-fetoprotein gene in the mouse. Proc Natl Acad Sci USA 1980; 77: 1351–1355.
Lu S-Y, Sui Y-F, Li Z-S, Pan C-E, Ye J, Wang W-Y . Construction f a regulable gene therapy vector targeting for hepatocellular carcinoma. World J Gastroenterol 2003; 9: 688–691.
Shi Y-J, Gong J-P, Liu C-A, Li X-H, Mei Y, Mi C et al. Construction of a targeting adenoviral vector carrying AFP promoter for expressing EGFP gene in AFP-producing hepatocarcinoma cell. World J Gastroenterol 2004; 10: 186–189.
Sa Cunha AS, Bonte E, Dubois S, Chretien Y, Eraiser T, Degott C et al. Inhibition of rat hepatocellular carcinoma tumor growth after multiple infusions of recombinant Ad. AFPtk followed by ganciclovir treatment. J Hepatol 2002; 37: 222–230.
Ido A, Uto H, Moriuchi A, Nagata K, Onaga Y, Onaga M et al. Gene therapy targeting for hepatocellular carcinoma: Selective and enhanced suicide gene expression regulated by a hypoxia-inducible enhancer linked to a human α-fetoprotein promoter. Cancer Res 2001; 61: 3016–3021.
Zhang D-E, Rabek JP, Hsieh C-C, Torres-Ramos C, Papaconstantinou J . Functional analysis of the mouse α-fetoprotein enhancers and their subfragments in primary mouse hepatocyte cultures. J Biol Chem 1992; 267: 10676–10682.
Arturi F, Russo D, Schlumberger M, Du Villard J-A, Caillou B, Vigneri P et al. Iodide symporter gene expression in human thyroid tumors. J Clin Endocrinol Metab 1998; 83: 2493–2496.
Castro MR, Bergert ER, Beito TG, McIver BD, Goellner JR, Morris JC . Development of monoclonal antibodies against the human sodium iodide symporter: Immunohistochemical characterization of this protein in thyroid cells. J Clin Endocrinol Metab 1999; 84: 2957–2962.
Caillou B, Troalen F, Baudin E, Talbot M, Filetti S, Schlumberger M et al. Na+/I− symporter distribution in human thyroid tissues: an immunohistochemical study. J Clin Endocrinol Metab 1998; 83: 4102–4106.
Dohan O, Baloch Z, Banrevi Z, Livolsi V, Carrasco N . Predominant intracellular overexpression of the Na+/I- symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab 2001; 86: 2697–2700.
Jhiang SM, Cho J-Y, Ryu K-Y, De Young BR, Smanik PA, McGaughy VR et al. An immunohistochemical study of Na+/I- symporter in human thyroid tissues and salivary gland tissues. Endocrinology 1998; 139: 4416–4419.
Zhang Z, Liu Y-Y, Jhiang SM . Cell surface targeting accounts for the difference in iodide uptake activity between human Na/I symporter and rat Na/I symporter. J Clin Endocrinol Metab 2005; 90: 6131–6140.
Dohan O, De la Vieja A, Carrasco N . Hydrocortisone and purinergic signaling stimulate NIS-mediated iodide transport in breast cancer cells. Mol Endocrinol 2006; 20: 1121–1137.
Dingli D, Peng K-W, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004; 103: 1641–1646.
Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC . Sodium iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Therapy 2006; 13: 60–66.
Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ, Morris JC . Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors. Hum Gene Ther 2006; 17: 661–668.
Faivre J, Clerc J, Gerolami R, Herve J, Longuet M, Liu B et al. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats. Cancer Res 2004; 64: 8045–8051.
Chen L, Altmann A, Mier W, Eskerski H, Leotta K, Guo L et al. Radioiodine therapy of hepatoma using targeted transfer of the human sodium/iodide symporter gene. J Nucl Med 2006; 47: 854–862.
Weiss SJ, Philip NJ, Grollmann EF . Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology 1984; 114: 1090–1098.
Urabe M, Hershman JM, Pang X-P, Murakami S, Sugawara M . Effect of lithium on function and growth of thyroid cells in vitro. Endocrinology 1991; 129: 807–814.
Mandell RB, Mandell LZ, Link CJ . Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res 1999; 59: 661–668.
Acknowledgements
We are grateful to Dr SM Jhiang, Ohio State University, Columbus, OH, USA, for supplying the full-length human NIS cDNA. This study was supported by grants to C Spitzweg (Sp/581/3-2, Sp 581/4-1, Sp 581/4-2 (DFG-Forschergruppe ‘Radionuklidtherapie’ FOR-411) from the German Research Council (Deutsche Forschungsgemeinschaft, Bonn, Germany), by the FöFoLe-Program of the Ludwig-Maximilians-University Munich to MJ Willhauck (FöFoLe Reg. Nr. 442), and by the Mayo Foundation Prostate Cancer SPORE grant (CA91956) to JC Morris.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willhauck, M., Sharif Samani, B., Klutz, K. et al. α-Fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther 15, 214–223 (2008). https://doi.org/10.1038/sj.gt.3303057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303057
Keywords
This article is cited by
-
The sodium iodide symporter (NIS) as theranostic gene: potential role in pre-clinical therapy of extra-thyroidal malignancies
Clinical and Translational Imaging (2023)
-
Dual-targeted NIS polyplexes—a theranostic strategy toward tumors with heterogeneous receptor expression
Gene Therapy (2019)
-
Sequence-defined cMET/HGFR-targeted Polymers as Gene Delivery Vehicles for the Theranostic Sodium Iodide Symporter (NIS) Gene
Molecular Therapy (2016)
-
Combination of hepatocyte specific delivery and transformation dependent expression of shRNA inducing transcriptional gene silencing of c-Myc promoter in hepatocellular carcinoma cells
BMC Cancer (2014)
-
Sodium iodide symporter (NIS) in extrathyroidal malignancies: focus on breast and urological cancer
BMC Cancer (2014)