Abstract
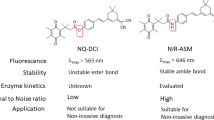
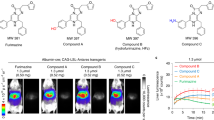
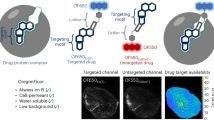
Development of nonimmunogenic and specific reporter genes to monitor gene expression in vivo is important for the optimization of gene therapy protocols. We developed a membrane-anchored form of mouse β-glucuronidase (mβG) as a reporter gene to hydrolyze a nonfluorescent glucuronide probe (fluorescein di-β-D-glucuronide, (FDGlcU) to a highly fluorescent reporter to assess the location and persistence of gene expression. A functional β-glucuronidase (βG) was stably expressed on the surface of murine CT26 colon adenocarcinoma cells where it selectively hydrolyzed the cell-impermeable FDGlcU probe. FDGlcU was also preferentially converted to fluorescent probe by (βG) on CT26 tumors. The fluorescent intensity in βG-expressing CT26 tumors was 240 times greater than the intensity in control tumors. Selective imaging of gene expression was also observed after intratumoral injection of adenoviral βG vector into carcinoma xenografts. Importantly, mβG did not induce an antibody response after hydrodynamic plasmid immunization of Balb/c mice, indicating that the reporter gene product displayed low immunogenicity. A membrane-anchored form of human βG also allowed in vivo imaging, demonstrating that human βG can be employed for imaging. This imaging system therefore, displays good selectivity with low immunogenicity and may help assess the location, magnitude and duration of gene expression in living animals and humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Misteli T, Spector DL . Applications of the green fluorescent protein in cell biology and biotechnology. Nat Biotechnol 1997; 15: 961–964.
Yang M, Baranov E, Jiang P, Sun FX, Li XM, Li L et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci USA 2000; 97: 1206–1211.
Colin M, Moritz S, Schneider H, Capeau J, Coutelle C, Brahimi-Horn MC . Haemoglobin interferes with the ex vivo luciferase luminescence assay: consequence for detection of luciferase reporter gene expression in vivo. Gene Therapy 2000; 7: 1333–1336.
Liang Q, Nguyen K, Satyamurthy N, Barrio JR, Phelps ME, Gambhir SS et al. Monitoring adenoviral DNA delivery, using a mutant herpes simplex virus type 1 thymidine kinase gene as a PET reporter gene. Gene Therapy 2002; 9: 1659–1666.
Haberkorn U, Oberdorfer F, Gebert J, Morr I, Haack K, Weber K et al. Monitoring gene therapy with cytosine deaminase: in vitro studies using tritiated-5-fluorocytosine. J Nucl Med 1996; 37: 87–94.
Yu J, Liu L, Kodibagkar VD, Cui W, Mason RP . Synthesis and evaluation of novel enhanced gene reporter molecules: Detection of beta-galactosidase activity using (19)F NMR of trifluoromethylated aryl beta-d-galactopyranosides. Bioorg Med Chem 2006; 14: 326–333.
Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997; 276: 1719–1724.
Haack K, Linnebacher M, Eisold S, Zoller M, von Knebel Doeberitz M, Gebert J . Induction of protective immunity against syngeneic rat cancer cells by expression of the cytosine deaminase suicide gene. Cancer Gene Ther 2000; 7: 1357–1364.
Russo V, Tanzarella S, Dalerba P, Rigatti D, Rovere P, Villa A et al. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci USA 2000; 97: 2185–2190.
Reichel MB, Bainbridge J, Baker D, Thrasher AJ, Bhattacharya SS, Ali RR . An immune response after intraocular administration of an adenoviral vector containing a beta galactosidase reporter gene slows retinal degeneration in the rd mouse. Br J Ophthalmol 2001; 85: 341–344.
MacLaren DC, Gambhir SS, Satyamurthy N, Barrio JR, Sharfstein S, Toyokuni T et al. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Therapy 1999; 6: 785–791.
Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA et al. In vivo magnetic resonance imaging of transgene expression. Nat Med 2000; 6: 351–355.
Wolf G . Vitamin A functions in the regulation of the dopaminergic system in the brain and pituitary gland. Nutr Rev 1998; 56: 354–355.
Fleming RE, Migas MC, Holden CC, Waheed A, Britton RS, Tomatsu S et al. Transferrin receptor 2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis. Proc Natl Acad Sci USA 2000; 97: 2214–2219.
Aleksandrowicz J, Starek A, Moszczynski P, Czarnobilski Z . Changes in the activity of lysosome beta-glucuronidase in rat leukocytes treated with various doses of selenium. Patol Pol 1978; 29: 143–151.
Stahl PD, Fishman WH . β- D-glucuronidase. In: Bergmeyer J, Graβl M (eds). Methods in Enzymatic Analysis. Verlag Chemie: Weinheim, 1984; vol 5. pp 246–256.
Haisma HJ, Boven E, van Muijen M, de Jong J, van der Vijgh WJ, Pinedo HM . A monoclonal antibody-beta-glucuronidase conjugate as activator of the prodrug epirubicin-glucuronide for specific treatment of cancer. Br J Cancer 1992; 66: 474–478.
Cheng TL, Chou WC, Chen BM, Chern JW, Roffler SR . Characterization of an antineoplastic glucuronide prodrug. Biochem Pharmacol 1999; 58: 325–328.
Kiang TK, Ensom MH, Chang TK . UDP-glucuronosyltransferases and clinical drug–drug interactions. Pharmacol Ther 2005; 106: 97–132.
Yu Y, Annala AJ, Barrio JR, Toyokuni T, Satyamurthy N, Namavari M et al. Quantification of target gene expression by imaging reporter gene expression in living animals. Nat Med 2000; 6: 933–937.
Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 2004; 22: 589–594.
Chou WC, Liao KW, Lo YC, Jiang SY, Yeh MY, Roffler SR . Expression of chimeric monomer and dimer proteins on the plasma membrane of mammalian cells. Biotechnol Bioeng 1999; 65: 160–169.
Cheng TL, Liao KW, Tzou SC, Cheng CM, Chen BM, Roffler SR . Hapten-directed targeting to single-chain antibody receptors. Cancer Gene Ther 2004; 11: 380–388.
Liao KW, Lo YC, Roffler SR . Activation of lymphocytes by anti-CD3 single-chain antibody dimers expressed on the plasma membrane of tumor cells. Gene Therapy 2000; 7: 339–347.
Liao KW, Chen BM, Liu TB, Tzou SC, Lin YM, Lin KF et al. Stable expression of chimeric anti-CD3 receptors on mammalian cells for stimulation of antitumor immunity. Cancer Gene Ther 2003; 10: 779–790.
Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R . In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer Res 2004; 64: 1579–1583.
Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol 2000; 18: 321–325.
Duimstra JA, Femia FJ, Meade TJ . A gadolinium chelate for detection of beta-glucuronidase: a self-immolative approach. J Am Chem Soc 2005; 127: 12847–12855.
Cheng TL, Chen BM, Chan LY, Wu PY, Chern JW, Roffler SR . Poly(ethylene glycol) modification of beta-glucuronidase-antibody conjugates for solid-tumor therapy by targeted activation of glucuronide prodrugs. Cancer Immunol Immunother 1997; 44: 305–315.
Chen BM, Chan LY, Wang SM, Wu MF, Chern JW, Roffler SR . Cure of malignant ascites and generation of protective immunity by monoclonal antibody-targeted activation of a glucuronide prodrug in rats. Int J Cancer 1997; 73: 392–402.
Cheng TL, Wei SL, Chen BM, Chern JW, Wu MF, Liu PW et al. Bystander killing of tumour cells by antibody-targeted enzymatic activation of a glucuronide prodrug. Br J Cancer 1999; 79: 1378–1385.
Leu YL, Roffler SR, Chern JW . Design and synthesis of water-soluble glucuronide derivatives of camptothecin for cancer prodrug monotherapy and antibody-directed enzyme prodrug therapy (ADEPT). J Med Chem 1999; 42: 3623–3628.
Cheng TL, Chen BM, Chern JW, Wu MF, Roffler SR . Efficient clearance of poly(ethylene glycol)-modified immunoenzyme with anti-PEG monoclonal antibody for prodrug cancer therapy. Bioconjug Chem 2000; 11: 258–266.
Chen BM, Cheng TL, Tzou SC, Roffler SR . Potentiation of antitumor immunity by antibody-directed enzyme prodrug therapy. Int J Cancer 2001; 94: 850–858.
de Graaf M, Pinedo HM, Oosterhoff D, van der Meulen-Muileman IH, Gerritsen WR, Haisma HJ et al. Pronounced antitumor efficacy by extracellular activation of a doxorubicin-glucuronide prodrug after adenoviral vector-mediated expression of a human antibody-enzyme fusion protein. Hum Gene Ther 2004; 15: 229–238.
Heine D, Muller R, Brusselbach S . Cell surface display of a lysosomal enzyme for extracellular gene-directed enzyme prodrug therapy. Gene Therapy 2001; 8: 1005–1010.
Chen KC, Cheng TL, Leu YL, Prijovich ZM, Chuang CH, Chen BM et al. Membrane-localized activation of glucuronide prodrugs by β-glucuronidase enzymes. Cancer Gene Ther 2006, September 15; [E-pub ahead of print].
Shyue SK, Tsai MJ, Liou JY, Willerson JT, Wu KK . Selective augmentation of prostacyclin production by combined prostacyclin synthase and cyclooxygenase-1 gene transfer. Circulation 2001; 103: 2090–2095.
Lin KJ, Ye XX, Yen TC, Wey SP, Tzen KY, Ting G et al. Biodistribution study of [(123)I] ADAM in mice: correlation with whole body autoradiography. Nucl Med Biol 2002; 29: 643–650.
Roffler SR, Wang HE, Yu HM, Chang WD, Cheng CM, Lu YL et al. A membrane antibody receptor for noninvasive imaging of gene expression. Gene Therapy 2005; 13: 412–420.
Andrianaivo F, Lecocq M, Wattiaux-De Coninck S, Wattiaux R, Jadot M . Hydrodynamics-based transfection of the liver: entrance into hepatocytes of DNA that causes expression takes place very early after injection. J Gene Med 2004; 6: 877–883.
Acknowledgements
This work was supported by the National Research Program for Genomic Medicine (NRPGM), National Science Council, Taipei, Taiwan (NSC95-3112-B-037-001 and NSC94-2745-B-037-010-URD) and the Genomic and Proteomic Program, Academia Sinica, Taipei, Taiwan (94M007-2). The Molecular-Genetic Imaging Core-Cell and tissue-imaging core of National Yang-Ming University is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Su, YC., Chuang, KH., Wang, YM. et al. Gene expression imaging by enzymatic catalysis of a fluorescent probe via membrane-anchored β-glucuronidase. Gene Ther 14, 565–574 (2007). https://doi.org/10.1038/sj.gt.3302896
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302896
Keywords
This article is cited by
-
Real-time imaging of intestinal bacterial β-glucuronidase activity by hydrolysis of a fluorescent probe
Scientific Reports (2017)
-
Modular assembly of synthetic proteins that span the plasma membrane in mammalian cells
BMC Biotechnology (2016)
-
Expression of β-glucuronidase on the surface of bacteria enhances activation of glucuronide prodrugs
Cancer Gene Therapy (2013)
-
Bacterial glucuronidase as general marker for oncolytic virotherapy or other biological therapies
Journal of Translational Medicine (2011)
-
Enhancement of CPT-11 antitumor activity by adenovirus-mediated expression of β–glucuronidase in tumors
Cancer Gene Therapy (2011)