Abstract
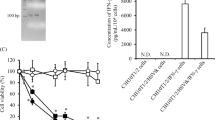
Ovarian cancer represents a malignancy suitable for cell and gene therapy approaches owing to its containment within the peritoneal cavity, even at advanced tumor stages. As regulation of transgene expression would be preferable for conducting clinical trials for reasons of safety, we investigated whether intraperitoneal (i.p.) administration of retroviral vector-transduced fibroblasts encoding murine interferon-α (IFN-α) could have therapeutic activity, and compared its effect with the antitumor effects of fibroblasts producing IFN-α under a rapamycin analogue (AP21967)-inducible promoter. Human and murine fibroblasts were recruited into the solid component of transplantable ovarian cancer-grown i.p. in severe combined immunodeficiency mice. Multiple administrations of fibroblasts producing IFN-α in a constitutive manner showed therapeutic efficacy, leading to significant prolongation of survival in the majority of animals, associated with inhibition of tumor angiogenesis. Compared to cells transduced by the constitutive vector, fibroblasts transduced by the inducible vector released twofold higher IFN-α levels in vitro, following induction by AP21967, and production of the cytokine was under pharmacologic control both in vitro and in vivo. However, these cells elicited only modest therapeutic effects in vivo. Overall, these findings indicate that intracavitary IFN-α gene therapy using engineered fibroblasts requires sustained production of IFN-α to achieve durable antitumor effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pisani P, Parkin DM, Bray F, Ferlay J . Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999; 83: 18–29.
Kirby TO, Curiel DT, Alvarez RD . Gene therapy for ovarian cancer: progress and potential. Hematol Oncol Clin N Am 2003; 17: 1021–1050.
Zeimet AG, Marth C . Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol 2003; 4: 415–422.
Roni V, Habeler W, Parenti A, Indraccolo S, Gola E, Tosello V et al. Recruitment of human umbilical vein endothelial cells and human primary fibroblasts into experimental tumors growing in SCID mice. Exp Cell Res 2003; 287: 28–38.
Sanches R, Kuiper M, Penault-Llorca F, Aunoble B, D'Incan C, Bignon YJ . Antitumoral effect of interleukin-12-secreting fibroblasts in a mouse model of ovarian cancer: implications for the use of ovarian cancer biopsy-derived fibroblasts as a vehicle for regional gene therapy. Cancer Gene Ther 2000; 7: 707–720.
Albini A, Marchisone C, Del Grosso F, Benelli R, Masiello L, Tacchetti C et al. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: a gene therapy approach. Am J Pathol 2000; 156: 1381–1393.
Indraccolo S, Gola E, Rosato A, Minuzzo S, Habeler W, Tisato V et al. Differential effects of angiostatin, endostatin and interferon-alpha(1) gene transfer on in vivo growth of human breast cancer cells. Gene Therapy 2002; 9: 867–878.
De Bouard S, Guillamo JS, Christov C, Lefevre N, Brugieres P, Gola E et al. Antiangiogenic therapy against experimental glioblastoma using genetically engineered cells producing interferon-alpha, angiostatin, or endostatin. Hum Gene Ther 2003; 14: 883–895.
Tuting T, Gambotto A, Baar J, Davis ID, Storkus WJ, Zavodny PJ et al. Interferon-alpha gene therapy for cancer: retroviral transduction of fibroblasts and particle-mediated transfection of tumor cells are both effective strategies for gene delivery in murine tumor models. Gene Therapy 1997; 4: 1053–1060.
Tahara H, Zeh III HJ, Storkus WJ, Pappo I, Watkins SC, Gubler U et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res 1994; 54: 182–189.
Tahara H, Lotze MT, Robbins PD, Storkus WJ, Zitvogel L . IL-12 gene therapy using direct injection of tumors with genetically engineered autologous fibroblasts. Hum Gene Ther 1995; 6: 1607–1624.
Shawler DL, Dorigo O, Gjerset RA, Royston I, Sobol RE, Fakhrai H . Comparison of gene therapy with interleukin-2 gene modified fibroblasts and tumor cells in the murine CT-26 model of colorectal carcinoma. J Immunother Emphasis Tumor Immunol 1995; 17: 201–208.
Zitvogel L, Tahara H, Robbins PD, Storkus WJ, Clarke MR, Nalesnik MA et al. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol 1995; 155: 1393–1403.
Lotze MT, Rubin JT, Carty S, Edington H, Ferson P, Landreneau R et al. Gene therapy of cancer: a pilot study of IL-4-gene-modified fibroblasts admixed with autologous tumor to elicit an immune response. Hum Gene Ther 1994; 5: 41–55.
Belardelli F, Ferrantini M, Proietti E, Kirkwood JM . Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13: 119–134.
Tough DF, Borrow P, Sprent J . Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 1996; 272: 1947–1950.
Pollock R, Issner R, Zoller K, Natesan S, Rivera VM, Clackson T . Delivery of a stringent dimerizer-regulated gene expression system in a single retroviral vector. Proc Natl Acad Sci USA 2000; 97: 13221–13226.
Bjornsti MA, Houghton PJ . The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4: 335–348.
Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 2002; 8: 128–135.
Marie I, Durbin JE, Levy DE . Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J 1998; 17: 6660–6669.
Roth DA, Tawa Jr NE, O'Brien JM, Treco DA, Selden RF . Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med 2001; 344: 1735–1742.
Kang WK, Park C, Yoon HL, Kim WS, Yoon SS, Lee MH et al. Interleukin 12 gene therapy of cancer by peritumoral injection of transduced autologous fibroblasts: outcome of a phase I study. Hum Gene Ther 2001; 12: 671–684.
Qin XQ, Tao N, Dergay A, Moy P, Fawell S, Davis A et al. Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc Natl Acad Sci USA 1998; 95: 14411–14416.
Ye X, Rivera VM, Zoltick P, Cerasoli Jr F, Schnell MA, Gao G et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science 1999; 283: 88–91.
Rowinsky EK . Targeting the molecular target of rapamycin (mTOR). Curr Opin Oncol 2004; 16: 564–575.
Rivera VM, Ye X, Courage NL, Sachar J, Cerasoli Jr F, Wilson JM et al. Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci USA 1999; 96: 8657–8662.
Dutcher JP . Mammalian target of rapamycin inhibition. Clin Cancer Res 2004; 10: 6382S–6387S.
Guba M, Yezhelyev M, Eichhorn ME, Schmid G, Ischenko I, Papyan A et al. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood 2005; 105: 4463–4469.
Dvorak HF, Gresser I . Microvascular injury in pathogenesis of interferon-induced necrosis of subcutaneous tumors in mice. J Natl Cancer Inst 1989; 81: 497–502.
Rozera C, Carlei D, Lollini PL, De Giovanni C, Musiani P, Di Carlo E et al. Interferon (IFN)-beta gene transfer into TS/A adenocarcinoma cells and comparison with IFN-alpha: differential effects on tumorigenicity and host response. Am J Pathol 1999; 154: 1211–1222.
Klein D, Indraccolo S, von Rombs K, Amadori A, Salmons B, Gunzburg WH . Rapid identification of viable retrovirus-transduced cells using the green fluorescent protein as a marker. Gene Therapy 1997; 4: 1256–1260.
Indraccolo S, Habeler W, Tisato V, Stievano L, Piovan E, Tosello V et al. Gene transfer in ovarian cancer cells: a comparison between retroviral and lentiviral vectors. Cancer Res 2002; 62: 6099–6107.
Lacerda JF, Ladanyi M, Jagiello C, O'Reilly RJ . Administration of rabbit anti-asialo GM1 antiserum facilitates the development of human Epstein–Barr virus-induced lymphoproliferations in xenografted C.B-17 scid/scid mice. Transplantation 1996; 61: 492–497.
Indraccolo S, Minuzzo S, Roccaforte F, Zamarchi R, Habeler W, Stievano L et al. Effects of CD2 locus control region sequences on gene expression by retroviral and lentiviral vectors. Blood 2001; 98: 3607–3617.
De Giovanni C, Palmieri G, Nicoletti G, Landuzzi L, Scotlandi K, Bontadini A et al. Immunological and non-immunological influence of H-2Kb gene transfection on the metastatic ability of B16 melanoma cells. Int J Cancer 1991; 48: 270–276.
Indraccolo S, Morini M, Gola E, Carrozzino F, Habeler W, Minghelli S et al. Effects of angiostatin gene transfer on functional properties and in vivo growth of Kaposi's sarcoma cells. Cancer Res 2001; 61: 5441–5446.
Acknowledgements
We thank Mrs A Azzalini and P Gallo for artwork and Dr Erich Piovan for help in preparing the manuscript. We thank ARIAD Pharmaceuticals Inc. (Cambridge, MA, USA) for providing us with the Argent Regulated Transcription Retrovirus Kit and with AP21967. This work was supported in part by grants from MIUR 40 and 60%, FIRB, the Italian Association for Cancer Research (AIRC), the Ministero della Salute (Ricerca Finalizzata 2002), the Italian Foundation for Cancer Research (FIRC), and the Fondazione Cassa di Risparmio di Padova e Rovigo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indraccolo, S., Moserle, L., Tisato, V. et al. Gene therapy of ovarian cancer with IFN-α-producing fibroblasts: comparison of constitutive and inducible vectors. Gene Ther 13, 953–965 (2006). https://doi.org/10.1038/sj.gt.3302745
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302745
Keywords
This article is cited by
-
Functional genomics of endothelial cells treated with anti-angiogenic or angiopreventive drugs
Clinical & Experimental Metastasis (2010)
-
Altered gene expression and miRNA expression associated with cancerous IEC-6 cell transformed by MNNG
Journal of Experimental & Clinical Cancer Research (2009)
-
Preclinical study of an ex vivo gene therapy protocol for hepatocarcinoma
Cancer Gene Therapy (2009)