Abstract
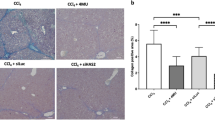
Hepatic fibrosis represents a process of healing and scarring in response to chronic liver injury. α-Melanocyte-stimulating hormone (α-MSH) is a 13-amino-acid peptide with potent anti-inflammatory effects. We have previously demonstrated that α-MSH gene therapy protects against thioacetamide (TAA)-induced acute liver failure. Therefore, the aim of this study is to investigate whether α-MSH gene therapy possesses antihepatic fibrogenic effect. Liver fibrosis was induced by long-term TAA administration in mice. α-Melanocyte-stimulating hormone expression plasmid was delivered via electroporation after liver fibrosis was established. Our results showed that α-MSH gene therapy attenuated liver fibrosis in TAA-treated mice. Reverse transcription polymerase chain reaction revealed that α-MSH gene therapy attenuated the liver transforming growth factor-β1, collagen α1 and cell adhesion molecule mRNA upregulation. Following gene transfer, the expression of α-smooth muscle actin and cyclooxygenase-2 were both significantly attenuated. Further, α-MSH significantly increased matrix metalloproteinase (MMP), while tissue inhibitors of matrix metalloproteinase (TIMPs) were inactivated. In summary, α-MSH gene therapy reversed established liver fibrosis in mice and prevented the upregulated fibrogenic and pro-inflammatory gene responses after TAA administration. Its collagenolytic effect might be attributed to MMP and TIMP modulation. Hence, α-MSH gene therapy may be an effective therapeutic modality against liver fibrosis with potential clinical use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wang CH, Chen YJ, Lee TH, Chen YS, Jawan B, Hung KS et al. Protective effect of MDL28170 against thioacetamide-induced acute liver failure in mice. J Biomed Sci 2004; 11: 571–578.
Wang CH, Jawan B, Lee TH, Hung KS, Chou WY, Lu CN et al. Single injection of naked plasmid encoding α-melanocyte-stimulating hormone protects against thioacetamide-induced acute liver failure in mice. Biochem Biophys Res Commun 2004; 322: 153–161.
Hung KS, Lee TH, Chou WY, Wu CL, Cho CL, Lu CN et al. Interleukin-10 gene therapy reverses thioacetamide-induced liver fibrosis in mice. Biochem Biophys Res Commun 2005; 336: 324–331.
Chieli E, Malvaldi G . Role of the microsomal FAD-containing monooxygenase in the liver toxicity of thioacetamide S-oxide. Toxicology 1984; 31: 41–52.
Hunter AL, Holscher MA, Neal RA . Thioacetamide-induced hepatic necrosis. I. Involvement of the mixed-function oxidase enzyme system. J Pharmacol Exp Ther 1977; 200: 439–448.
Porter WR, Gudzinowicz MJ, Neal RA . Thioacetamide-induced hepatic necrosis. II. Pharmacokinetics of thioacetamide and thioacetamide-S-oxide in the rat. J Pharmacol Exp Ther 1979; 208: 386–391.
Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I et al. Melatonin inhibits nuclear factor κB activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J Hepatol 2004; 40: 86–93.
Tunez I, Munoz MC, Villavicencio MA, Medina FJ, de Prado EP, Espejo I et al. Hepato- and neurotoxicity induced by thioacetamide: protective effects of melatonin and dimethylsulfoxide. Pharmacol Res 2005; 52: 223–228.
Poli G . Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med 2000; 21: 49–98.
Reeves HL, Friedman SL . Activation of hepatic stellate cells – a key issue in liver fibrosis. Front Biosci 2002; 7: D808–D826.
Schuppan D, Ruehl M, Somasundaram R, Hahn EG . Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis 2001; 21: 351–372.
Desmet VJ, Roskams T . Cirrhosis reversal: a duel between dogma and myth. J Hepatol 2004; 40: 860–867.
Gressner AM, Weiskirchen R, Breitkopf K, Dooley S . Roles of TGF-β in hepatic fibrosis. Front Biosci 2002; 7: D793–D807.
Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology 1992; 103: 264–274.
Catania A, Airaghi L, Colombo G, Lipton JM . α-Melanocyte-stimulating hormone in normal human physiology and disease states. Trends Endocrinol Metab 2000; 11: 304–308.
Luger TA, Scholzen TE, Brzoska T, Bohm M . α-New insights into the functions of α-MSH and related peptides in the immune system. Ann NY Acad Sci 2003; 994: 133–140.
Chiao H, Foster S, Thomas R, Lipton J, Star RA . α-Melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Invest 1996; 97: 2038–2044.
Bohm M, Raghunath M, Sunderkotter C, Schiller M, Stander S, Brzoska T et al. Collagen metabolism is a novel target of the neuropeptide α-melanocyte-stimulating hormone. J Biol Chem 2004; 279: 6959–6966.
Colombo G, Gatti S, Turcatti F, Sordi A, Fassati LR, Bonino F et al. Gene expression profiling reveals multiple protective influences of the peptide α-melanocyte-stimulating hormone in experimental heart transplantation. J Immunol 2005; 175: 3391–3401.
Lee SY, Jo SK, Cho WY, Kim HK, Won NH . The effect of α-melanocyte-stimulating hormone on renal tubular cell apoptosis and tubulointerstitial fibrosis in cyclosporine A nephrotoxicity. Transplantation 2004; 78: 1756–1764.
Kiss M, Wlaschek M, Brenneisen P, Michel G, Hommel C, Lange TS et al. α-Melanocyte-stimulating hormone induces collagenase/matrix metalloproteinase-1 in human dermal fibroblasts. Biol Chem Hoppe Seyler 1995; 376: 425–430.
Lei TC, Vieira WD, Hearing VJ . In vitro migration of melanoblasts requires matrix metalloproteinase-2: implications to vitiligo therapy by photochemotherapy. Pigment Cell Res 2002; 15: 426–432.
Wilson JF, Harry FM . Release, distribution and half-life of α-melanotrophin in the rat. J Endocrinol 1980; 86: 61–67.
Schnur J, Olah J, Szepesi A, Nagy P, Thorgeirsson SS . Thioacetamide-induced hepatic fibrosis in transforming growth factor β-1 transgenic mice. Eur J Gastroenterol Hepatol 2004; 16: 127–133.
Rothuizen J, Biewenga WJ, Mol JA . Chronic glucocorticoid excess and impaired osmoregulation of vasopressin release in dogs with hepatic encephalopathy. Domest Anim Endocrinol 1995; 12: 13–24.
Claria J . Cyclooxygenase-2 biology. Curr Pharm Des 2003; 9: 2177–2190.
Yamamoto H, Kondo M, Nakamori S, Nagano H, Wakasa K, Sugita Y et al. JTE-522, a cyclooxygenase-2 inhibitor, is an effective chemopreventive agent against rat experimental liver fibrosis. Gastroenterology 2003; 125: 556–571.
Mohammed NA, Abd El-Aleem SA, El-Hafiz HA, McMahon RF . Distribution of constitutive (COX-1) and inducible (COX-2) cyclooxygenase in postviral human liver cirrhosis: a possible role for COX-2 in the pathogenesis of liver cirrhosis. J Clin Pathol 2004; 57: 350–354.
Sung YK, Hwang SY, Kim JO, Bae HI, Kim JC, Kim MK . The correlation between cyclooxygenase-2 expression and hepatocellular carcinogenesis. Mol Cells 2004; 17: 35–38.
Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG et al. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res 2001; 7: 1410–1418.
Cheng J, Imanishi H, Liu W, Iwasaki A, Ueki N, Nakamura H et al. Inhibition of the expression of α-smooth muscle actin in human hepatic stellate cell line, LI90, by a selective cyclooxygenase 2 inhibitor, NS-398. Biochem Biophys Res Commun 2002; 297: 1128–1134.
Shiratori K, Ohgami K, Ilieva IB, Koyama Y, Yoshida K, Ohno S . Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by α-melanocyte-stimulating hormone. Invest Ophthalmol Vis Sci 2004; 45: 159–164.
Caruso C, Mohn C, Karara AL, Rettori V, Watanobe H, Schioth HB et al. α-Melanocyte-stimulating hormone through melanocortin-4 receptor inhibits nitric oxide synthase and cyclooxygenase expression in the hypothalamus of male rats. Neuroendocrinology 2004; 79: 278–286.
Maga G, Hubscher U . Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 2003; 116: 3051–3060.
Jeong DH, Jang JJ, Lee SJ, Lee JH, Lim IK, Lee MJ et al. Expression patterns of cell cycle-related proteins in a rat cirrhotic model induced by CCl4 or thioacetamide. J Gastroenterol 2001; 36: 24–32.
Donato MF, Arosio E, Del Ninno E, Ronchi G, Lampertico P, Morabito A et al. High rates of hepatocellular carcinoma in cirrhotic patients with high liver cell proliferative activity. Hepatology 2001; 34: 523–528.
Giron-Gonzalez JA, Martinez-Sierra C, Rodriguez-Ramos C, Rendon P, Macias MA, Fernandez-Gutierrez C et al. Adhesion molecules as a prognostic marker of liver cirrhosis. Scand J Gastroenterol 2005; 40: 217–224.
Burra P, Hubscher SG, Shaw J, Elias E, Adams DH . Is the intercellular adhesion molecule-1/leukocyte function associated antigen 1 pathway of leukocyte adhesion involved in the tissue damage of alcoholic hepatitis? Gut 1992; 33: 268–271.
Simeonova PP, Gallucci RM, Hulderman T, Wilson R, Kommineni C, Rao M et al. The role of tumor necrosis factor-α in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol 2001; 177: 112–120.
Ip E, Farrell G, Hall P, Robertson G, Leclercq I . Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 2004; 39: 1286–1296.
Scholzen TE, Sunderkotter C, Kalden DH, Brzoska T, Fastrich M, Fisbeck T et al. Melanocyte-stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression. Endocrinology 2003; 144: 360–370.
Arthur MJ . Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2000; 279: G245–G249.
Del Carmen Garciade Leon M, Montfort I, Tello Montes E, Lopez Vancell R, Olivos Garcia A, Gonzalez Canto A et al. Hepatocyte production of modulators of extracellular liver matrix in normal and cirrhotic rat liver. Exp Mol Pathol 2006; 80: 97–108.
Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A et al. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology 2004; 40: 1106–1115.
Xu GF, Li PT, Wang XY, Jia X, Tian DL, Jiang LD et al. Dynamic changes in the expression of matrix metalloproteinases and their inhibitors, TIMPs, during hepatic fibrosis induced by alcohol in rats. World J Gastroenterol 2004; 10: 3621–3627.
Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 2004; 126: 1795–1808.
Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK et al. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology 2003; 124: 445–458.
Siller-Lopez F, Sandoval A, Salgado S, Salazar A, Bueno M, Garcia J et al. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology 2004; 126: 1122–1133.
Limb GA, Matter K, Murphy G, Cambrey AD, Bishop PN, Morris GE et al. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am J Pathol 2005; 166: 1555–1563.
Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ . Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol 2001; 7: 363–369.
Yin P, Luby TM, Chen H, Etemad-Moghadam B, Lee D, Aziz N et al. Generation of expression constructs that secrete bioactive α-MSH and their use in the treatment of experimental autoimmune encephalomyelitis. Gene Therapy 2003; 10: 348–355.
Molnar MJ, Gilbert R, Lu Y, Liu AB, Guo A, Larochelle N et al. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol Ther 2004; 10: 447–455.
Vergnes L, Phan J, Strauss M, Tafuri S, Reue K . Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem 2003; 278: 42774–42784.
Young DA, Lakey RL, Pennington CJ, Jones D, Kevorkian L, Edwards DR et al. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther 2005; 7: R503–R512.
Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL et al. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol 1998; 153: 1895–1902.
Acknowledgements
This work was supported by Chang Gung Memorial Hospital Research Grant No. 840451 (CMRPG-840451). We thank Dr Hedley for providing us the recombinant α-MSH expression plasmid used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, CH., Lee, TH., Lu, CN. et al. Electroporative α-MSH gene transfer attenuates thioacetamide-induced murine hepatic fibrosis by MMP and TIMP modulation. Gene Ther 13, 1000–1009 (2006). https://doi.org/10.1038/sj.gt.3302744
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302744
Keywords
This article is cited by
-
Anti-fibrotic effect of PF2401-SF, a standardized fraction of Salvia miltiorrhiza, in thioacetamide-induced experimental rats liver fibrosis
Archives of Pharmacal Research (2015)
-
Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated α-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells
Gene Therapy (2007)