Abstract
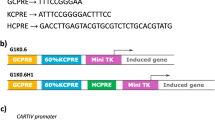
Although a significant negative prognostic factor, tumor hypoxia can be exploited for gene therapy. To maximize targeting within the tumor mass, we have developed synthetic gene promoters containing hypoxia-responsive elements (HREs) from the erythropoietin (Epo) gene as well as radiation-responsive CArG elements from the early growth response (Egr) 1 gene. Furthermore, to achieve high and sustained expression of the suicide gene herpes simplex virus thymidine kinase (HSVtk), our gene therapy vectors contain an expression amplification system, or ‘molecular switch’, based on Cre/loxP recombination. In human glioma and breast adenocarcinoma cells exposed to hypoxia and/or radiation, the HRE/CArG promoter rapidly activated Cre recombinase expression leading to selective and sustained HSVtk synthesis. Killing of transfected tumor cells was measured after incubation with the prodrug ganciclovir (GCV; converted by HSVtk into a cytotoxin). In vitro, higher and more selective GCV-mediated toxicity was achieved with the switch vectors, when compared with the same inducible promoters driving HSVtk expression directly. In tumor xenografts implanted in nude mice, the HRE/CArG-switch induced significant growth delay and tumor eradication. In conclusion, hypoxia- and radiation-activated ‘molecular switch’ vectors represent a promising strategy for both targeted and effective gene therapy of solid tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Knisely JP, Rockwell S . Importance of hypoxia in the biology and treatment of brain tumors. Neuroimaging Clin N Am 2002; 12: 525–536.
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P . Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–4515.
Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Greenberg RE, Stobbe C et al. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology 2002; 60: 634–639.
Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW . Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997; 38: 285–289.
Brown JM, Giaccia AJ . The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998; 58: 1408–1416.
Hill SA, Pigott KH, Saunders MI, Powell ME, Arnold S, Obeid A et al. Microregional blood flow in murine and human tumours assessed using laser Doppler microprobes. Br J Cancer Suppl 1996; 27: S260–S263.
Raleigh JA, Calkins-Adams DP, Rinker LH, Ballenger CA, Weissler MC, Fowler Jr WC et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res 1998; 58: 3765–3768.
Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol 1998; 48: 149–156.
Brizel DM, Dodge RK, Clough RW, Dewhirst MW . Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 1999; 53: 113–117.
Hockel M, Schlenger K, Hockel S, Vaupel P . Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res 1999; 59: 4525–4528.
Hockel M, Vaupel P . Biological consequences of tumor hypoxia. Semin Oncol 2001; 28: 36–41.
Greco O, Marples B, Joiner MC, Scott SD . How to overcome (and exploit) tumor hypoxia for targeted gene therapy. J Cell Physiol 2003; 197: 312–325.
Semenza GL, Wang GL . A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992; 12: 5447–5454.
Beck I, Weinmann R, Caro J . Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood 1993; 82: 704–711.
Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ et al. Targeting gene expression to hypoxic tumor cells. Nat Med 1997; 3: 515–520.
Shibata T, Giaccia AJ, Brown JM . Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Therapy 2000; 7: 493–498.
Greco O, Marples B, Dachs GU, Williams KJ, Patterson AV, Scott SD . Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Therapy 2002; 9: 1403–1411.
Scott SD, Marples B, Hendry JH, Lashford LS, Embleton MJ, Hunter RD et al. A radiation-controlled molecular switch for use in gene therapy of cancer. Gene Therapy 2000; 7: 1121–1125.
Kaczmarczyk SJ, Green JE . A single vector containing modified cre recombinase and LOX recombination sequences for inducible tissue-specific amplification of gene expression. Nucleic Acids Res 2001; 29 (12, e56): 1–13.
Salloum RM, Mauceri HJ, Hanna NN, Gorski DH, Posner MC, Weichselbaum RR . Dual induction of the Epo-Egr-TNF-alpha- plasmid in hypoxic human colon adenocarcinoma produces tumor growth delay. Am Surg 2003; 69: 24–27.
Elshami AA, Saavedra A, Zhang H, Kucharczuk JC, Spray DC, Fishman GI et al. Gap junctions play a role in the ‘bystander effect’ of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Therapy 1996; 3: 85–92.
Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL et al. The ‘bystander effect’: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 1993; 53: 5274–5283.
Gagandeep S, Brew R, Green B, Christmas SE, Klatzmann D, Poston GJ et al. Prodrug-activated gene therapy: involvement of an immunological component in the ‘bystander effect’. Cancer Gene Ther 1996; 3: 83–88.
Vile RG, Castleden S, Marshall J, Camplejohn R, Upton C, Chong H . Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intratumoural cytokine expression. Int J Cancer 1997; 71: 267–274.
Zhang W, Couldwell WT, Simard MF, Song H, Lin JH, Nedergaard M . Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res 1999; 59: 1994–2003.
Scott SD, Joiner MC, Marples B . Optimizing radiation-responsive gene promoters for radiogenetic cancer therapy. Gene Therapy 2002; 9: 1396–1402.
Durand RE, Raleigh JA . Identification of nonproliferating but viable hypoxic tumor cells in vivo. Cancer Res 1998; 58: 3547–3550.
Hillman GG, Xu M, Wang Y, Wright JL, Lu X, Kallinteris NL et al. Radiation improves intratumoral gene therapy for induction of cancer vaccine in murine prostate carcinoma. Hum Gene Ther 2003; 14: 763–775.
Acknowledgements
We are very grateful to Drs George Wilson and Michael Borrelli for scientific discussion, and to Dr Yu Wang, Ms Sara Bourne, Mr Evano Piasentin and Ms Jennifer Wright for excellent technical support. This work was supported by The Susan G Komen Breast Cancer Foundation, Wayne State University, Academic Radiation Oncologists and Radiation Oncology Research & Development Center, Detroit, MI, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greco, O., Joiner, M., Doleh, A. et al. Hypoxia- and radiation-activated Cre/loxP ‘molecular switch’ vectors for gene therapy of cancer. Gene Ther 13, 206–215 (2006). https://doi.org/10.1038/sj.gt.3302640
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302640
Keywords
This article is cited by
-
Detailed assessment of gene activation levels by multiple hypoxia-responsive elements under various hypoxic conditions
Annals of Nuclear Medicine (2014)
-
Evaluation of p21 promoter for interleukin 12 radiation induced transcriptional targeting in a mouse tumor model
Molecular Cancer (2013)
-
The radiation dose-regulated AND gate genetic circuit, a novel targeted and real-time monitoring strategy for cancer gene therapy
Cancer Gene Therapy (2012)
-
Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis
Gene Therapy (2009)
-
Translation of the radio- and chemo-inducible TNFerade vector to the treatment of human cancers
Cancer Gene Therapy (2009)