Abstract
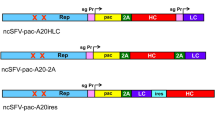
The efficient gene transfer of immunostimulatory cytokines into autologous tumor cells or the transfer of tumor-associated antigens into professional antigen-presenting cells is a prerequisite for many immunotherapeutic approaches. In particular with B cells, the efficiency of gene uptake is one of the limiting factors in cell-based vaccine strategies, since normal and malignant human B cells are commonly refractory to transducing gene vectors. Due to its natural tropism for human B cells, Epstein–Barr virus (EBV), a human herpes virus, might be an option, which we wanted to explore. EBV efficiently infects human B cells and establishes a latent infection, while the viral genome is maintained extrachromosomally. Although these characteristics are attractive, EBV is an oncogenic virus. Here, we present a novel EBV-derived vector, which lacks three EBV genes including two viral oncogenes and an essential lytic gene, and encodes granulocyte-macrophage colony-stimulating factor (GM-CSF) as a cytokine of therapeutic interest. We could show that EBV vectors efficiently transduce different B-cell lines, primary resting B cells, and tumor cells of B-cell lineage. Vector-derived GM-CSF was expressed in sufficient amounts to support the maturation of dendritic cells and their presentation of model antigens to cognate T-cell clones in autologous settings and an allogeneic, HLA-matched assay. We conclude that the EBV vector system might offer an option for ex vivo manipulation of B cells and gene therapy of B-cell lymphomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pardoll DM . Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol 2002; 2: 227–238.
Kay MA, Glorioso JC, Naldini L . Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 2001; 7: 33–40.
Rieger R, Kipps TJ . CpG oligodeoxynucleotides enhance the capacity of adenovirus-mediated CD154 gene transfer to generate effective B-cell lymphoma vaccines. Cancer Res 2003; 63: 4128–4135.
Kofler DM, Buning H, Mayr C, Bund D, Baumert J, Hallek M et al. Engagement of the B-cell antigen receptor (BCR) allows efficient transduction of ZAP-70-positive primary B-CLL cells by recombinant adeno-associated virus (rAAV) vectors. Gene Therapy 2004; 11: 1416–1424.
Anonymous. Epstein–Barr Virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. Lyon: International Agency for Research on Cancer (IARC), 1997.
Hammerschmidt W, Sugden B . Genetic analysis of immortalizing functions of Epstein–Barr virus in human B lymphocytes. Nature 1989; 340: 393–397.
Dirmeier U, Neuhierl B, Kilger E, Reisbach G, Sandberg ML, Hammerschmidt W . Latent membrane protein 1 is critical for efficient growth transformation of human B cells by Epstein–Barr virus. Cancer Res 2003; 63: 2982–2989.
Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W et al. The Epstein–Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J 2000; 19: 3080–3089.
Schepers A, Pich D, Hammerschmidt W . A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of Epstein–Barr virus. Eur Mol Biol Org J 1993; 12: 3921–3929.
Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W . Propagation and recovery of intact, infectious Epstein–Barr virus from prokaryotic to human cells. Proc Natl Acad Sci USA 1998; 95: 8245–8250.
Metcalf D, Burgess AW, Johnson GR, Nicola NA, Nice EC, DeLamarter J et al. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF. J Cell Physiol 1986; 128: 421–431.
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 1993; 90: 3539–3543.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature 1998; 392: 245–252.
Dranoff G . GM-CSF-secreting melanoma vaccines. Oncogene 2003; 22: 3188–3192.
Simons JW . Bioactivity of human GM-CSF gene therapy in metastatic renal cell carcinoma and prostate cancer. Hinyokika Kiyo 1997; 43: 821–822.
Dranoff G, Soiffer R, Lynch T, Mihm M, Jung K, Kolesar K et al. A phase I study of vaccination with autologous, irradiated melanoma cells engineered to secrete human granulocyte-macrophage colony stimulating factor. Hum Gene Ther 1997; 8: 111–123.
Soiffer R, Hodi FS, Haluska F, Jung K, Gillessen S, Singer S et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol 2003; 21: 3343–3350.
Mahvi DM, Sondel PM, Yang NS, Albertini MR, Schiller JH, Hank J et al. Phase I/IB study of immunization with autologous tumor cells transfected with the GM-CSF gene by particle-mediated transfer in patients with melanoma or sarcoma. Hum Gene Ther 1997; 8: 875–891.
Nemunaitis J, Sterman D, Jablons D, Smith II JW, Fox B, Maples P et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Nat Cancer Inst 2004; 96: 326–331.
Delecluse HJ, Pich D, Hilsendegen T, Baum C, Hammerschmidt W . A first-generation packaging cell line for Epstein–Barr virus-derived vectors. Proc Natl Acad Sci USA 1999; 96: 5188–5193.
Delecluse HJ, Hammerschmidt W . The genetic approach to the Epstein–Barr virus: from basic virology to gene therapy. Mol Pathol 2000; 53: 270–279.
Kieff E, Rickinson AB . Epstein–Barr virus and its replication. In: Knipe DM et al. (eds.), Field's Virology. Philadelphia: Lippincott-Williams & Wilkins, 2001, pp. 2511–2573.
Kilger E, Kieser A, Baumann M, Hammerschmidt W . Epstein–Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J 1998; 17: 1700–1709.
Dirmeier U, Hoffmann R, Kilger E, Schultheiss U, Briseño C, Gires O et al. Latent membrane protein 1 of Epstein–Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene 2005; 24: 1711–1717.
Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N . Expression of the Epstein–Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA 1998; 95: 11963–11968.
Wilson JB, Weinberg W, Johnson R, Yuspa S, Levine AJ . Expression of the BNLF-1 oncogene of Epstein–Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell 1990; 61: 1315–1327.
Wang D, Liebowitz D, Kieff E . An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985; 43: 831–840.
Cohen JI, Wang F, Mannick J, Kieff E . Epstein–Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci USA 1989; 86: 9558–9562.
Karasuyama H, Kudo A, Melchers F . The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med 1990; 172: 969–972.
Neuhierl B, Feederle R, Delecluse HJ . Glycoprotein gp110 of Epstein–Barr virus determines viral tropism and efficiency of infection. Proc Natl Acad Sci USA 2002; 99: 15036–15041.
Sugden B, Mark W . Clonal transformation of adult human leukocytes by Epstein–Barr virus. J Virol 1977; 23: 503–508.
Klausner RD, Donaldson JG, Lippincott-Schwartz J . Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 1992; 116: 1071–1080.
Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M et al. An Epstein–Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 1991; 5: 147–156.
Goldblum N, Daefler S, Llana T, Ablashi D, Josephs S, Salahuddin Z . Susceptibility to HIV-1 infection of a human B-lymphoblastoid cell line, DG75, transfected with subgenomic DNA fragments of Epstein–Barr virus. Dev Biol Stand 1990; 72: 309–313.
Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol 1989; 140: 323–334.
Nimmerjahn F, Kobelt D, Steinkasserer A, Menke A, Hobom G, Behrends U et al. Efficient generation and expansion of antigen-specific CD4+ T cells by recombinant influenza viruses. Eur J Immunol 2003; 33: 3331–3341.
Sallusto F, Cella M, Danieli C, Lanzavecchia A . Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 1995; 182: 389–400.
Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J et al. Murine leukemia induced by retroviral gene marking. Science 2002; 296: 497.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80: 148–158.
Kreppel F, Kochanek S . Long-term transgene expression in proliferating cells mediated by episomally maintained high-capacity adenovirus vectors. J Virol 2004; 78: 9–22.
Wendtner CM, Kofler DM, Mayr C, Bund D, Hallek M . The potential of gene transfer into primary B-CLL cells using recombinant virus vectors. Leuk Lymphoma 2004; 45: 897–904.
Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ . CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 2000; 96: 2917–2924.
Janssens W, Chuah MK, Naldini L, Follenzi A, Collen D, Saint-Remy JM et al. Efficiency of onco-retroviral and lentiviral gene transfer into primary mouse and human B-lymphocytes is pseudotype dependent. Hum Gene Therapy 2003; 14: 263–276.
Bonamino M, Serafini M, D'Amico G, Gaipa G, Todisco E, Bernasconi S et al. Functional transfer of CD40L gene in human B-cell precursor ALL blasts by second-generation SIN lentivectors. Gene Therapy 2004; 11: 85–93.
Lizee G, Gonzales MI, Topalian SL . Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Therapy 2004; 15: 393–404.
Spender LC, Cannell EJ, Hollyoake M, Wensing B, Gawn JM, Brimmell M et al. Control of cell cycle entry and apoptosis in B lymphocytes infected by Epstein–Barr virus. J Virol 1999; 73: 4678–4688.
Conese M, Auriche C, Ascenzioni F . Gene therapy progress and prospects: episomally maintained self-replicating systems. Gene Therapy 2004; 11: 1735–1741.
Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA 1998; 95: 13141–13146.
Strehl J, Selmayr M, Kremer JP, Hultner L, Lindhofer H, Mocikat R . Gene therapy of B-cell lymphoma with cytokine gene-modified trioma cells. Int J Cancer 1999; 83: 113–120.
Selmayr M et al. Induction of tumor immunity by autologous B lymphoma cells expressing a genetically engineered idiotype. Gene Therapy 1999; 6: 778–784.
Mach N, Dranoff G . Cytokine-secreting tumor cell vaccines. Curr Opin Immunol 2000; 12: 571–575.
Levitsky HI et al. Immunization with granulocyte-macrophage colony-stimulating factor-transduced, but not B7-1-transduced, lymphoma cells primes idiotype-specific T cells and generates potent systemic antitumor immunity. J Immunol 1996; 156: 3858–3865.
Maeda A et al. Epstein–Barr virus can infect B-chronic lymphocytic leukemia cells but it does not orchestrate the cell cycle regulatory proteins. J Hum Virol 2001; 4: 227–237.
Teramoto N et al. Epstein–Barr virus-infected B-chronic lymphocyte leukemia cells express the virally encoded nuclear proteins but they do not enter the cell cycle. J Hum Virol 2000; 3: 125–136.
Wahl U et al. Vaccination against B-cell chronic lymphocytic leukemia with trioma cells: preclinical evaluation. Clin Cancer Res 2003; 9: 4240–4246.
Posfai G, Koob MD, Kirkpatrick HA, Blattner FR . Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J Bacteriol 1997; 179: 4426–4428.
O'Connor M, Peifer M, Bender W . Construction of large DNA segments in Escherichia coli. Science 1989; 244: 1307–1312.
Cherepanov PP, Wackernagel W . Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1995; 158: 9–14.
Hammerschmidt W, Sugden B . Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein–Barr virus. Cell 1988; 55: 427–433.
Schultheiss U et al. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J 2001; 20: 5678–5691.
Nimmerjahn F et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol 2003; 33: 1250–1259.
Acknowledgements
This work was supported by the Sanderstiftung to RM and WH, by Grants Ha1354/3 and SFB455 of the Deutsche Forschungsgemeinschaft, and by Public Health Service Grant CA70723 to WH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellebrand, E., Mautner, J., Reisbach, G. et al. Epstein–Barr virus vector-mediated gene transfer into human B cells: potential for antitumor vaccination. Gene Ther 13, 150–162 (2006). https://doi.org/10.1038/sj.gt.3302602
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302602
Keywords
This article is cited by
-
Activated B cells in autoimmune diseases: the case for a regulatory role
Nature Clinical Practice Rheumatology (2008)
-
Genetic design of an optimized packaging cell line for gene vectors transducing human B cells
Gene Therapy (2006)