Abstract
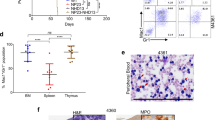
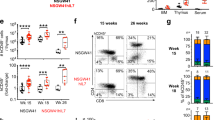
Defects in the gene for the IL-7 receptor (R) α chain are one cause of severe combined immunodeficiency disease (SCID) based on a strict requirement for IL-7 in T lymphoid development and survival. We tested the feasibility and potentially undesirable consequences of IL-7Rα gene transfer as a therapy for this genetic defect. The murine IL-7Rα gene was introduced into IL-7Rα−/− bone marrow progenitors using retrovirus and transplanted into Rag−/− recipient mice. Both αβ and γδ T cells were reconstituted in thymus and spleen showing proof of principle. B-cell development was also restored in some mice, but their numbers were much lower than in the T-cell compartment. Splenomegaly was observed due to an increase in neutrophils. We showed that hematopoeitic progenitors, after transfection with IL-7Rα, could respond to IL-7 in vitro by a striking production of neutrophils and other myeloid cells. These data indicate that although IL-7 is a critical lymphopoietin, ectopic expression of its receptor on multipotential progenitors can also induce production of myeloid cells, presumably through survival and proliferation signals that are not restricted to lymphoid cells. This supports the stochastic model of progenitor differentiation, in which cytokines give permissive and not instructive signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hofmeister R et al. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev 1999; 10: 41–60.
Puel A, Ziegler SF, Buckley RH, Leonard WJ . Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet 1998; 20: 394–397.
Roifman CM, Zhang J, Chitayat D, Sharfe N . A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood 2000; 96: 2803–2807.
Noguchi M et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science 1993; 262: 1877–1880.
Macchi P et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 1995; 377: 65–68.
Grabstein KH et al. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med 1993; 178: 257–264.
Peschon JJ et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 1994; 180: 1955–1960.
von-Freeden-Jeffry U et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 1995; 181: 1519–1526.
Akashi K et al. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 1997; 89: 1033–1041.
Kim K et al. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol 1998; 160: 5735–5741.
Maraskovsky E et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 1997; 89: 1011–1019.
Khaled AR et al. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA 1999; 96: 14476–14481.
Khaled AR et al. Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity 2002; 17: 561–573.
Durum SK et al. Interleukin 7 receptor control of T cell receptor gamma gene rearrangement: role of receptor-associated chains and locus accessibility. J Exp Med 1998; 188: 2233–2241.
Brugnera E et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity 2000; 13: 59–71.
Schluns KS, Kieper WC, Jameson SC, Lefrancois L . Interleukin-7 mediates the homeostasis of naive and memory CD8T cells in vivo. Nat Immunol 2000; 1: 426–432.
Tan JT et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8(+) cells but are not required for memory phenotype CD4(+) cells. J Exp Med 2002; 195: 1523–1532.
Kalman L et al. Mutations in genes required for T-cell development: IL7R, CD45, IL2RG, JAK3, RAG1, RAG2, ARTEMIS, and ADA and severe combined immunodeficiency: HuGE review. Genet Med 2004; 6: 16–26.
Cavazzana-Calvo M et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672.
Hacein-Bey-Abina S et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Jiang Q et al. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol 2004; 24: 6501–6513.
Hacein-Bey H et al. Gamma-c gene transfer into SCID X1 patients’ B-cell lines restores normal high-affinity interleukin-2 receptor expression and function. Blood 1996; 87: 3108–3116.
Kohn DB et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med 1995; 1: 1017–1023.
Rolink A et al. Immature surface Ig+ B cells can continue to rearrange kappa and lambda L chain gene loci. J Exp Med 1993; 178: 1263–1270.
Purohit SJ et al. Determination of lymphoid cell fate is dependent on the expression status of the IL-7 receptor. EMBO J 2003; 22: 5511–5521.
Kaech SM et al. Selective expression of the interleukin 7 receptor identifies effector CD8T cells that give rise to long-lived memory cells. Nat Immunol 2003; 4: 1191–1198.
Opferman JT et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003; 426: 671–676.
Lopez AF et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest 1986; 78: 1220–1228.
Goldsmith MA et al. Absence of cytokine receptor-dependent specificity in red blood cell differentiation in vivo. Proc Natl Acad Sci USA 1998; 95: 7006–7011.
Dubart A et al. Murine pluripotent hematopoietic progenitors constitutively expressing a normal erythropoietin receptor proliferate in response to erythropoietin without preferential erythroid cell differentiation. Mol Cell Biol 1994; 14: 4834–4842.
Pharr PN, Ogawa M, Hofbauer A, Longmore GD . Expression of an activated erythropoietin or a colony-stimulating factor 1 receptor by pluripotent progenitors enhances colony formation but does not induce differentiation. Proc Natl Acad Sci USA 1994; 91: 7482–7486.
Nishijima I et al. A human GM-CSF receptor expressed in transgenic mice stimulates proliferation and differentiation of hemopoietic progenitors to all lineages in response to human GM-CSF. Mol Biol Cell 1995; 6: 497–508.
Yang FC et al. Human granulocyte colony-stimulating factor (G-CSF) stimulates the in vitro and in vivo development but not commitment of primitive multipotential progenitors from transgenic mice expressing the human G-CSF receptor. Blood 1998; 92: 4632–4640.
Semerad CL, Poursine-Laurent J, Liu F, Link DC . A role for G-CSF receptor signaling in the regulation of hematopoietic cell function but not lineage commitment or differentiation. Immunity 1999; 11: 153–161.
Takagi M et al. Multi-colony stimulating activity of interleukin 5 (IL-5) on hematopoietic progenitors from transgenic mice that express IL-5 receptor alpha subunit constitutively. J Exp Med 1995; 181: 889–899.
Damia G et al. Administration of recombinant human interleukin-7 alters the frequency and number of myeloid progenitor cells in the bone marrow and spleen of mice. Blood 1992; 79: 1121–1129.
Grzegorzewski K et al. Administration of recombinant human interleukin-7 to mice induces the exportation of myeloid progenitor cells from the bone marrow to peripheral sites. Blood 1994; 83: 377–385.
Dave UP, Jenkins NA, Copeland NG . Gene therapy insertional mutagenesis insights. Science 2004; 303: 333.
Nolan GP, Shatzman AR . Expression vectors and delivery systems. Curr Opin Biotechnol 1998; 9: 447–450.
Acknowledgements
We thank R Wyles for technical assistance, K Noer for flow cytometry and J Oppenheim for comments on the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jiang, Q., Li, WQ., Aiello, F. et al. Retroviral transduction of IL-7Rα into IL-7Rα−/− bone marrow progenitors: correction of lymphoid deficiency and induction of neutrophilia. Gene Ther 12, 1761–1768 (2005). https://doi.org/10.1038/sj.gt.3302558
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302558
Keywords
This article is cited by
-
Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges
Nature Reviews Drug Discovery (2019)
-
Correction of murine Rag1 deficiency by self-inactivating lentiviral vector-mediated gene transfer
Leukemia (2011)
-
Thymopoiesis in elderly human is associated with systemic inflammatory status
AGE (2009)
-
Interleukin-7 receptor expression: intelligent design
Nature Reviews Immunology (2007)