Abstract
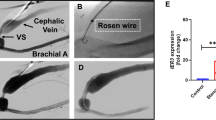
Gene transfer to the vessel wall using vascular endothelial growth factors (VEGFs) has shown therapeutic potential for the treatment of restenosis. In this study, we evaluated the effect of catheter-mediated adenoviral (Ad) gene transfer of the mature form of VEGF-D (VEGF-DΔNΔC) in balloon-denuded cholesterol-fed rabbit aorta. AdLacZ was used as a control. Transduced VEGF-DΔNΔC mRNA was detectable in the arterial wall with RT-PCR at 6, 14 and 28 days. Gene transfer efficiency as detected with X-gal staining 6 days after the AdLacZ transduction was 1.91±1.32% in intima. AdVEGF-DΔNΔC gene transfer led to 52% reduction in intima/media ratio (I/M) as compared to the AdLacZ controls at 14 days time point. At 6 days there were no differences in I/M, but the number of macrophages in the vessel wall was 85% lower in the AdVEGF-DΔNΔC group as compared to the controls. The therapeutic effect was no longer detectable 28 days after the gene transfer. The therapeutic effect of VEGF-DΔNΔC was nitric oxide (NO)-dependent as the feeding of NO synthase inhibitor, L-NAME, blocked the reduction in intimal thickening. It is concluded that AdVEGF-DΔNΔC gene transfer reduces intimal thickening and macrophage influx into the vessel wall in balloon-denuded rabbit aortas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others

References
Narins CR, Holmes DRJ, Topol EJ . A call for provisional stenting: the balloon is back!. Circulation 1998; 97: 1298–1305.
Zachary I, Mathur A, Yla-Herttuala S, Martin J . Vascular protection: a novel nonangiogenic cardiovascular role for vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 2000; 20: 1512–1520.
Ferrara N, Alitalo K . Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999; 5: 1359–1364.
Hiltunen MO et al. Intravascular adenovirus-mediated VEGF-C gene transfer reduces neointima formation in balloon-denuded rabbit aorta. Circulation 2000; 102: 2262–2268.
Laitinen M et al. Catheter-mediated vascular endothelial growth factor gene transfer to human coronary arteries after angioplasty. Hum Gene Ther 2000; 11: 263–270.
Makinen K et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther 2002; 6: 127–133.
Hedman M et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 2003; 107: 2677–2683.
Achen MG et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998; 95: 548–553.
Stacker SA et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem 1999; 274: 32127–32136.
Rutanen J et al. Vascular endothelial growth factor-D expression in human atherosclerotic lesions. Cardiovasc Res 2003; 59: 971–979.
Hiltunen MO et al. Biodistribution of adenoviral vector to nontarget tissues after local in vivo gene transfer to arterial wall using intravascular and periadventitial gene delivery methods. FASEB J 2000; 14: 2230–2236.
Rissanen TT et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res 2003; 92: 1098–1106.
Rutanen J et al. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation 2004; 109: 1029–1035.
Bauters C et al. Mechanisms and prevention of restenosis: from experimental models to clinical practice. Cardiovasc Res 1996; 31: 835–846.
Asahara T et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation 1995; 91: 2793–2801.
Ferrara N . Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999; 77: 527–543.
Sarkar R, Gordon D, Stanley JC, Webb RC . Dual cell cycle-specific mechanisms mediate the antimitogenic effects of nitric oxide in vascular smooth muscle cells. J Hypertens 1997; 15: 275–283.
Yan Z, Hansson GK . Overexpression of inducible nitric oxide synthase by neointimal smooth muscle cells. Circ Res 1998; 82: 21–29.
Kumar A, Lindner V . Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 1997; 17: 2238–2244.
Serrano Jr CV et al. Coronary angioplasty results in leukocyte and platelet activation with adhesion molecule expression. Evidence of inflammatory responses in coronary angioplasty. J Am Coll Cardiol 1997; 29: 1276–1283.
Kornowski R et al. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 1998; 31: 224–230.
Brasen JH et al. Angiogenesis, vascular endothelial growth factor and platelet-derived growth factor-BB expression, iron deposition, and oxidation-specific epitopes in stented human coronary arteries. Arterioscler Thromb Vasc Biol 2001; 21: 1720–1726.
Farb A et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation 2002; 105: 2974–2980.
Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R . Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res 1995; 76: 980–986.
Tsao PS et al. Nitric oxide regulates monocyte chemotactic protein-1. Circulation 1997; 96: 934–940.
De Caterina R et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995; 96: 60–68.
Qian H et al. Nitric oxide synthase gene therapy rapidly reduces adhesion molecule expression and inflammatory cell infiltration in carotid arteries of cholesterol-Fed rabbits. Circulation 1999; 99: 2979–2982.
Tsakiris DA et al. Circulating cell adhesion molecules and endothelial markers before and after transluminal angioplasty in peripheral arterial occlusive disease. Atherosclerosis 1999; 142: 193–200.
Cipollone F et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol 2001; 21: 327–334.
Laitinen M et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum Gene Ther 1998; 9: 1481–1486.
Leppanen O et al. Oral imatinib mesylate (STI571/gleevec) improves the efficacy of local intravascular vascular endothelial growth factor-C gene transfer in reducing neointimal growth in hypercholesterolemic rabbits. Circulation 2004; 109: 1140–1146.
Yla-Herttuala S, Alitalo K . Gene transfer as a tool to induce therapeutic vascular growth. Nat Med 2003; 9: 694–701.
Puumalainen AM et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther 1998; 9: 1769–1774.
Jones MK, Sarfeh IJ, Tarnawski AS . Induction of in vitro angiogenesis in the endothelial-derived cell line, EA hy926, by ethanol is mediated through PKC and MAPK. Biochem Biophys Res Commun 1998; 249: 118–123.
Laitinen M et al. Vegf gene transfer reduces intimal thickening via increased production of nitric oxide in carotid arteries. Hum Gene Ther 1997; 8: 1737–1744.
Ylä-Herttuala S et al. Transfer of 15-lipoxygenase gene into rabbit iliac arteries results in the appearance of oxidation-specific lipid-protein adducts characteristic of oxidized low density lipoprotein. J Clin Invest 1995; 95: 2692–2698.
Hakkinen T, Karkola K, Yla-Herttuala S . Macrophages, smooth muscle cells, endothelial cells, and T-cells express CD40 and CD40L in fatty streaks and more advanced human atherosclerotic lesions. Colocalization with epitopes of oxidized low-density lipoprotein, scavenger receptor, and CD16 (Fc gammaRIII). Virchows Arch 2000; 437: 396–405.
Acknowledgements
This study was supported by grants from the Finnish Academy, Sigrid Juselius Foundation, Ludwig Institute for Cancer Research, Finnish Medical Foundation, Finnish Cultural Foundation of Northern Savo, Research and Science Foundation of Farmos, Emil Aaltonen Foundation and Aarne Koskelo Foundation. SAS and MGA are supported by a Program Grant from the National Health and Medical Research Council of Australia (NHMRC), and Senior Research Fellowships from the Pharmacia Foundation and the NHMRC, respectively. We thank Mervi Nieminen, Liisa Korhonen, Tiina Koponen, Anna Tuomisto and Seija Sahrio for technical assistance and Boston Scientific for catheters.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rutanen, J., Turunen, AM., Teittinen, M. et al. Gene transfer using the mature form of VEGF-D reduces neointimal thickening through nitric oxide-dependent mechanism. Gene Ther 12, 980–987 (2005). https://doi.org/10.1038/sj.gt.3302489
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302489
Keywords
This article is cited by
-
Current Status of Cardiovascular Gene Therapy
Molecular Therapy (2007)
-
Mechanismen der arteriellen Restenose und Therapieansätze zur Prävention
Gefässchirurgie (2006)