Abstract
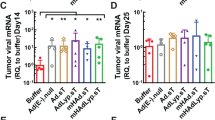
In clinical trials with cancer patients, the safety of conditionally replicating adenoviruses (CRAds) has been good. However, marginal data are available on the persistence or antitumor efficacy of these agents. The oncolytic potency of CRAds is determined by their capacity for entering target cells. Consequently, we constructed a retargeted CRAd featuring a secreted marker protein, soluble human carcinoembryogenic antigen (hCEA), which can be measured in growth medium or plasma. We found that virus replication closely correlated with hCEA secretion both in vitro and in vivo. Further, antitumor efficacy and the persistence of the virus could be deduced from plasma hCEA levels. Finally, using in vivo bioluminescence imaging, we were able to detect effective tumor cell killing by the virus, which led to enhanced therapeutic efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kirn D, Martuza RL, Zwiebel J . Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med 2001; 7: 781–787.
Hemminki A et al. Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res 2001; 61: 6377–6381.
Kanerva A et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther 2003; 8: 449–458.
Bauerschmitz GJ, Barker SD, Hemminki A . Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review). Int J Oncol 2002; 21: 1161–1174.
Kanerva A et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 2002; 8: 275–280.
Kanerva A et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 2002; 5: 695–704.
Segerman A et al. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J Virol 2003; 77: 1157–1162.
Segerman A et al. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol 2003; 77: 9183–9191.
Gaggar A, Shayakhmetov DM, Lieber A . CD46 is a cellular receptor for group B adenoviruses. Nat Med 2003; 9: 1408–1412.
Short JJ et al. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology 2004; 322: 349–359.
Fueyo J et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000; 19: 2–12.
Sherr CJ . Cancer cell cycles. Science 1996; 274: 1672–1677.
Peng KW et al. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med 2002; 8: 527–531.
Peng KW et al. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res 2002; 62: 4656–4662.
Panza N et al. Cancer antigen 125, tissue polypeptide antigen, carcinoembryonic antigen, and beta-chain human chorionic gonadotropin as serum markers of epithelial ovarian carcinoma. Cancer 1988; 61: 76–83.
Meier W et al. Significance of tumor marker determinations in the primary therapy of ovarian cancer. Anticancer Res 1997; 17: 2949–2951.
Horwitz MS . Adenovirus immunoregulatory genes and their cellular targets. Virology 2001; 279: 1–8.
Benedict CA et al. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J Biol Chem 2001; 276: 3270–3278.
Hawkins LK et al. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the 6.7K/gp19K region. Gene Therapy 2001; 8: 1123–1131.
Berinstein NL . Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol 2002; 20: 2197–2207.
Ehrhardt A, Xu H, Kay MA . Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J Virol 2003; 77: 7689–7695.
Gambhir SS, Barrio JR, Herschman HR, Phelps ME . Assays for noninvasive imaging of reporter gene expression. Nucl Med Biol 1999; 26: 481–490.
Min JJ, Gambhir SS . Gene therapy progress and prospects: noninvasive imaging of gene therapy in living subjects. Gene Therapy 2004; 11: 115–125.
Suzuki K, Alemany R, Yamamoto M, Curiel DT . The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res 2002; 8: 3348–3359.
Acknowledgements
This work was supported by the Sohlberg Foundation, Sigrid Juselius Foundation, Emil Aaltonen Foundation, Maud Kuistila Foundation, Finnish Medical Foundation Duodecim, Academy of Finland, Finnish Cancer Society, Biocentrum Helsinki, Biomedicum Helsinki Foundation, Ida Montin Foundation, University of Helsinki Internal Funds, HUCH Research Funds, Research and Science Foundation of Farmos, Instrumentarium Research Fund, an unrestricted grant from AstraZeneca, and the NIH grants (R01 CA94084, R01 CA83821, P50 CA83591, R01 CA93796).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanerva, A., Zinn, K., Peng, KW. et al. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene Ther 12, 87–94 (2005). https://doi.org/10.1038/sj.gt.3302387
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302387
Keywords
This article is cited by
-
Ad5/3 is able to avoid neutralization by binding to erythrocytes and lymphocytes
Cancer Gene Therapy (2021)
-
Biodistribution Analysis of Oncolytic Adenoviruses in Patient Autopsy Samples Reveals Vascular Transduction of Noninjected Tumors and Tissues
Molecular Therapy (2015)
-
Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4
Gene Therapy (2012)
-
Evaluation of adenovirus capsid labeling versus transgene expression
Virology Journal (2010)
-
Potent Oncolytic Activity of Raccoonpox Virus in the Absence of Natural Pathogenicity
Molecular Therapy (2010)