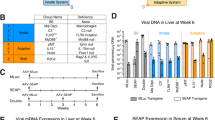
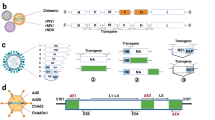
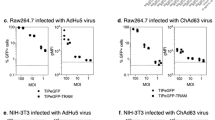
Abstract
Circumventing the immune response to the vector is a major challenge with all vector types. Viral vectors are the most likely to induce an immune response, especially those, like adenovirus and AAV, which express immunogenic epitopes within the organism. The first immune response occurring after vector transfer emerges from the innate immune system, mainly consisting in a rapid (few hours) inflammatory cytokines and chemokines secretion around the administration site. This reaction is high with adenoviral vectors and almost null with AAV. It is noteworthy that plasmid DNA vectors, because of CpG stimulatory islets, also stimulate the innate immunity via the stimulation of TLR receptors on leukocytes. Specific immune response leading to antibodies production and T lymphocytes activation also occurs within a few days after vector introduction. Capsid antigens are mostly responsible for specific immunity toward adenoviruses, and are also involved in the response against AAV. In the former case only, however, viral gene-encoded proteins can also be immunogenic. The pre-existing humoral immunity coming from early infections with wild-type AAV or adenovirus can prevent efficient gene transfer with the corresponding vectors. In all cases, some parameters like route of administration, dose, or promoter type have been extensively described as critical factors influencing vector immunity. Strategies to fight against vector-induced immunity can come from the immunology field, since tolerance induction or immunosuppression are a possibility. Alterations to vector structure have also been extensively performed to circumvent the immune system and thus enhance gene transfer efficiency and safety.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schauber CA et al. Lentiviral vectors pseudotyped with baculovirus gp64 efficiently transduce mouse cells in vivo and show tropism restriction against hematopoietic cell types in vitro. Gene Therapy 2004; 11: 266–275.
Wakimoto H et al. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Therapy 2003; 10: 983–990.
Worgall S et al. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 1997; 8: 37–44.
Liu Y et al. Rapid induction of cytotoxic T-cell response against cervical cancer cells by human papillomavirus type 16 E6 antigen gene delivery into human dendritic cells by an adeno-associated virus vector. Cancer Gene Ther 2001; 8: 948–957.
Zsengeller Z et al. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol 2000; 74: 9655–9667.
Rafii S et al. Infection of endothelium with E1(−)E4(+), but not E1(−)E4(−), adenovirus gene transfer vectors enhances leukocyte adhesion and migration by modulation of ICAM-1, VCAM-1, CD34, and chemokine expression. Circ Res 2001; 88: 903–910.
Schnell MA et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther 2001; 3: 708–722.
Lehrman S . Virus treatment questioned after gene therapy death. Nature 1999; 401: 517–518.
Zaiss AK et al. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol 2002; 76: 4580–4590.
Yasuda K et al. Plasmid DNA activates murine macrophages to induce inflammatory cytokines in a CpG motif-independent manner by complex formation with cationic liposomes. Biochem Biophys Res Commun 2002; 293: 344–348.
Yi AK, Krieg AM . Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J Immunol 1998; 161: 4493–4497.
Roman M et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med 1997; 3: 849–854.
Krug A et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol 2001; 31: 3026–3037.
Reyes-Sandoval A, Ertl HC . CpG methylation of a plasmid vector results in extended transgene product expression by circumventing induction of immune responses. Mol Ther 2004; 9: 249–261.
Paster W et al. In vivo plasmid DNA electroporation generates exceptionally high levels of epitope-specific CD8+ T-cell responses. Gene Therapy 2003; 10: 717–724.
Coelho-Castelo AA et al. B-lymphocytes in bone marrow or lymph nodes can take up plasmid DNA after intramuscular delivery. Hum Gene Ther 2003; 14: 1279–1285.
Chirmule N et al. Role of E4 in eliciting CD4 T-cell and B-cell responses to adenovirus vectors delivered to murine and nonhuman primate lungs. J Virol 1998; 72: 6138–6145.
Molinier-Frenkel V et al. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol 2000; 74: 7678–7682.
Kafri T et al. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA 1998; 95: 11377–11382.
Zhong L et al. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol 1999; 29: 964–972.
Zhang Y et al. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J Virol 2000; 74: 8003–8010.
Chiriva-Internati M et al. Testing recombinant adeno-associated virus-gene loading of dendritic cells for generating potent cytotoxic T lymphocytes against a prototype self-antigen, multiple myeloma HM1.24. Blood 2003; 102: 3100–3107; Epub 2003 Jul 3110.
Xin KQ et al. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum Gene Ther 2002; 13: 1571–1581.
Sarukhan A et al. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J Virol 2001; 75: 269–277.
Chirmule N et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Therapy 1999; 6: 1574–1583.
Smith TA et al. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Therapy 1996; 3: 496–502.
Cichon G et al. Complement activation by recombinant adenoviruses. Gene Therapy 2001; 8: 1794–1800.
Gahery-Segard H et al. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol 1998; 72: 2388–2397.
O'Riordan CR et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther 1999; 10: 1349–1358.
Erles K, Sebokova P, Schlehofer JR . Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999; 59: 406–411.
Moskalenko M et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol 2000; 74: 1761–1766.
Halbert CL et al. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol 1997; 71: 5932–5941.
Fisher KJ et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 1997; 3: 306–312.
Beck SE et al. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J Virol 1999; 73: 9446–9455.
Lo WD et al. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum Gene Ther 1999; 10: 201–213.
Mastakov MY et al. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol 2002; 76: 8446–8454.
Anand V et al. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol Ther 2002; 5: 125–132.
Cottard V et al. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol 2004; 24: 162–169.
Boyle MP et al. Effect of adeno-associated virus-specific immunoglobulin G in human amniotic fluid on gene transfer. Hum Gene Ther 2003; 14: 365–373.
Xiao W et al. Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999; 73: 3994–4003.
Hildinger M et al. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001; 75: 6199–6203.
Onodera M et al. Gene therapy for severe combined immunodeficiency caused by adenosine deaminase deficiency: improved retroviral vectors for clinical trials. Acta Haematol 1999; 101: 89–96.
Brockstedt DG et al. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol 1999; 92: 67–75.
Sun JY et al. Immune responses to adeno-associated virus and its recombinant vectors. Gene Therapy 2003; 10: 964–976.
Harding TC et al. Intravenous administration of an AAV-2 vector for the expression of factor IX in mice and a dog model of hemophilia B. Gene Therapy 2004; 11: 204–213.
Mingozzi F et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003; 111: 1347–1356.
Ge Y et al. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood 2001; 97: 3733–3737.
Halbert CL et al. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 1998; 72: 9795–9805.
Manning WC et al. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther 1998; 9: 477–485.
Song XY et al. Plasmid DNA encoding transforming growth factor-beta1 suppresses chronic disease in a streptococcal cell wall-induced arthritis model. J Clin Invest 1998; 101: 2615–2621.
Zhang YC et al. Immunity to adeno-associated virus serotype 2 delivered transgenes imparted by genetic predisposition to autoimmunity. Gene Therapy 2004; 11: 233–240.
Ritter T et al. Stimulatory and inhibitory action of cytokines on the regulation of hCMV-IE promoter activity in human endothelial cells. Cytokine 2000; 12: 1163–1170.
Harms JS, Splitter GA . Interferon-gamma inhibits transgene expression driven by SV40 or CMV promoters but augments expression driven by the mammalian MHC I promoter. Hum Gene Ther 1995; 6: 1291–1297.
Rabinowitz JE et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002; 76: 791–801.
Sarkar R et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood 2004; 103: 1253–1260; Epub 2003 Oct 1259.
Gao GP et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy.PG – 11854-9. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Seshidhar Reddy P et al. Development of adenovirus serotype 35 as a gene transfer vector. Virology 2003; 311: 384–393.
Schwartz RH . T cell anergy. Annu Rev Immunol 2003; 21: 305–334.
Kay MA et al. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet 1995; 11: 191–197.
Yang Y et al. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol 1996; 70: 6370–6377.
Banchereau J et al. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann NY Acad Sci 2003; 987: 180–187.
Moore KW et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765.
Abe M, Thomson AW . Influence of immunosuppressive drugs on dendritic cells. Transpl Immunol 2003; 11: 357–365.
Rea D et al. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood 2000; 95: 3162–3167.
Macian F et al. T-cell anergy. Curr Opin Immunol 2004; 16: 209–216.
Waddington SN et al. Reduced toxicity of F-deficient Sendai virus vector in the mouse fetus. Gene Therapy 2004; 11: 599–608.
Chen Y et al. Identification of methylated CpG motifs as inhibitors of the immune stimulatory CpG motifs. Gene Therapy 2001; 8: 1024–1032.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bessis, N., GarciaCozar, F. & Boissier, MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther 11 (Suppl 1), S10–S17 (2004). https://doi.org/10.1038/sj.gt.3302364
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302364
Keywords
This article is cited by
-
Where should siRNAs go: applicable organs for siRNA drugs
Experimental & Molecular Medicine (2023)
-
Prevalence of AAV2.5 neutralizing antibodies in synovial fluid and serum of patients with osteoarthritis
Gene Therapy (2023)
-
Review of gene therapies for age-related macular degeneration
Eye (2022)
-
Gene therapy for primary mitochondrial diseases: experimental advances and clinical challenges
Nature Reviews Neurology (2022)
-
In Utero Gene Editing for Inherited Lung Diseases
Current Stem Cell Reports (2022)