Abstract
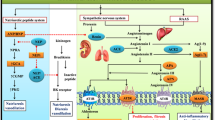
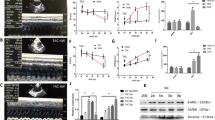
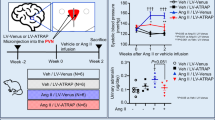
Gene therapy of hypertension requires long-term expression of a therapeutic gene to achieve stable reduction of blood pressure. Human tissue kallikrein (HK) cleaves kininogen to produce a potent vasoactive peptide kinin, which plays an important role in the regulation of the cardiovascular and renal functions. In the present study, we have delivered human kallikrein cDNA with an rAAV vector to explore the potential therapeutic effects of kallikrein on hypertension and related secondary complications. A single tail vein injection of the rAAV-HK vector into the adult spontaneously hypertensive rats resulted in a significant reduction (12.0±2.55 mmHg, P<0.05, n=6, ANOVA) of the systolic blood pressure from 2 weeks after vector injection, when compared with the control rAAV-lacZ vector-injected rats. Weekly blood pressure monitoring showed stable hypertension-reduction effect throughout the course of the 20-week experiments. In addition, total urine microalbumin contents decreased as a result of rAAV-HK treatment. Histological analysis of various tissues showed remarkable amelioration of cardiovascular hypertrophy, renal injury and collagen depositions in the rAAV-treated group. Finally, persistent expression of the transgene product HK was confirmed by the enzyme-linked immunosorbent assay and reverse transcription-polymerase chain reaction. We conclude that rAAV-mediated HK delivery rendered a long-term and stable reduction of hypertension and protected against renal injury, cardiac remodeling in the spontaneously hypertensive rat model. Further studies are warranted for the development of a gene therapy strategy for human hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Phillips MI . Is gene therapy for hypertension possible? Hypertension 1999; 33: 8–13.
Craig H et al. Current perspectives on the use of gene therapy for hypertension. Circ Res 2000; 87: 1118–1122.
Phillips MI . Gene therapy for hypertension: the preclinical data. Hypertension 2001; 38 (Part 2): 543–548.
Xiao X et al. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper virus. J Virol 1998; 72: 2224–2232.
Sun L, Li J, Xiao X . Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat Med 2000; 6: 599–602.
Monahan PE, Samulski RJ . AAV: is clinical success in the horizon? Gene Therapy 2000; 7: 24–30.
Margolius HS . Kallikreins and kinins. Some unanswered questions about system characteristics and roles in human disease. Hypertension 1995; 26: 221–229.
Kitamura N et al. Structural organization of the human kininogen gene and a model for its evolution. J Biol Chem 1985; 260: 8610–8617.
Müller-Esterl W, Iwanga S, Nakaniski S . Kininogens revisited. Trends Biochem Sci 1986; 11: 336–339.
Nakaniski S, Substance P . Precursor and kininogen: their structures, gene organizations, and regulation. Physiol Rev 1987; 67: 1117–1142.
Adam A et al. Human kininogens of low and high molecular mass: quantification by radioimmunoassay and determination of reference values. Clin Chem 1985; 31: 423–426.
Elliot AH, Nuzum FR . The urinary excretion of a depressor substance (kallikrein of Frey and Kraut) in arterial hypertension. Endocrinology 1934; 18: 462–474.
Zinner SH et al. Stability of blood pressure rank and urinary kallikrein concentration in childhood. Circulation 1978; 58: 908–915.
Margolius HS . Tissue kallikreins and kinins: regulation and roles in hypertensive and diabetic diseases. Ann Rev Pharmacol Toxicol 1989; 29: 343–364.
Berry TD et al. A gene for high urinary kallikrein may protect against hypertension in Utah kindreds. Hypertension 1989; 13: 3–8.
Majima M et al. High sensitivity to salt in kininogen-deficient Brown Norway Katholiek rats. Hypertension 1993; 22: 705–714.
Favaro S et al. Renal kallikrein content of spontaneously hypertensive rats. Clin Sci 1975; 49: 69–71.
Mayfield R et al. Development of hypertension in rats made diabetic with streptozotocin is associated with decreased urinary kallikrein excretion. Diabetes 1985; 34: 22–28.
Sharma JN, Uma K, Noor AR, Rahman AR . Blood pressure regulation by the kallikrein–kinin system. Gen Pharmacol 1996; 27: 55–63.
Katori M, Majima M . Pivotal role of renal kallikrein–kinin system in the development of hypertension and approaches to new drugs based on this relationship. Jpn Pharmacol 1996; 70: 95–128.
Mayfield RK, Margolius HS . Renal kallikrein–kinin system. Am J Nephrol 1983; 3: 145–155.
Chao J et al. Human kallikrein gene delivery attenuates hypertension, cardiac hypertrophy and renal injury in Dahl salt-sensitive rats. Hum Gene Ther 1998; 9: 21–31.
Chao J et al. Adenovirus-mediated kallikrein gene delivery reverses salt-induced renal injury in Dahl salt-sensitive rats. Kidney Int 1998; 54: 1250–1260.
Dobrzynski E et al. Adenovirus-mediated kallikrein gene delivery attenuates hypertension and protects against renal injury in deoxycorticosterone-salt rats. Immunopharmacology 1999; 44: 57–65.
Murakami H et al. Human kallikrein gene delivery protects against gentamycin-induced nephrotoxicity in rats. Kidney Int 1998; 53: 1305–1313.
Wolf WC et al. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney Int 2000; 58: 730–739.
Yayama K et al. Kallikrein gene delivery attenuates hypertension and cardiac hypertrophy and enhances renal function in Goldblatt hypertensive rats. Hypertension 1998; 31: 1104–1110.
Tolbert EM et al. Onset of glomerular hypertension with aging precedes injury in the spontaneously hypertensive rat. Am J Physiol Renal Physiol 2000; 278: F839–F846.
Francis SC et al. Genetic targeting for cardiovascular therapeutics: are we near the summit or just beginning the climb? Physiol Genomics 2001; 21: 79–94.
Linz W, Scholkens BA . Role of bradykinin in the cardiac effects of angiotensin-converting enzyme inhibitors. J Cardiovasc Pharmacol 1992; 20 (Suppl 9): 583–590.
Yoshida H et al. Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Hypertension 2000; 35: 25–31.
Monahan PE, Samulski RJ . Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today 2000; 6: 433–440.
Xiao X, Li J, Samulski RJ . Efficient long-term gene transfer into muscle tissue of immunocompetent mice with a rAAV vector. J Virol 1996; 70: 8098–8108.
Auricchio A et al. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001; 12: 71–76.
Xiong W, Chao J, Chao L . Muscle delivery of human kallikreingene reduces blood pressure in hypertensive rats. Hypertension 1995; 25: 715–719.
Guesdon JL, Ternynck T, Avrameas S . The use of avidin–biotin interaction in immunoenzymatic techniques. J Histochem Cytochem 1979; 27: 1131–1139.
Wang C et al. Adrenomedullin gene delivery attenuates renal damage and cardiac hypertrophy in Goldblatt hypertensive rats. Am J Physiol Renal Physiol 2001; 280: F964–F971.
Acknowledgements
This work was supported by the National ‘863’ Plan project of China (No. 2001AA217121) and International Collaboration Grant of Wuhan City (No. 997005135G).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, T., Li, H., Zhao, C. et al. Recombinant adeno-associated virus-mediated kallikrein gene therapy reduces hypertension and attenuates its cardiovascular injuries. Gene Ther 11, 1342–1350 (2004). https://doi.org/10.1038/sj.gt.3302294
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302294
Keywords
This article is cited by
-
Pancreatic kallikrein protects against diabetic retinopathy in KK Cg-Ay/J and high-fat diet/streptozotocin-induced mouse models of type 2 diabetes
Diabetologia (2019)
-
Activation of the ERK signaling pathway is involved in CD151-induced angiogenic effects on the formation of CD151-integrin complexes
Acta Pharmacologica Sinica (2010)
-
Recombinant adeno-associated virus-mediated human kallikrein gene therapy protects against hypertensive target organ injuries through inhibiting cell apoptosis
Acta Pharmacologica Sinica (2009)
-
Hypertension and the bradykinin system
Current Hypertension Reports (2009)
-
Packaging and functional identification of recombinant adeno-associated virus encoding cdc2-siRNA
Journal of Huazhong University of Science and Technology [Medical Sciences] (2008)