Abstract
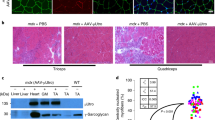
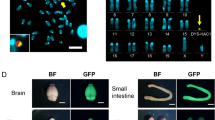
Gene therapy for Duchenne muscular dystrophy has so far not been successful because of the difficulty in achieving efficient and permanent gene transfer to the large number of affected muscles and the development of immune reactions against vector and transgenic protein. In addition, the prenatal onset of disease complicates postnatal gene therapy. We have therefore proposed a fetal approach to overcome these barriers. We have applied β-galactosidase expressing equine infectious anaemia virus (EIAV) lentiviruses pseudotyped with VSV-G by single or combined injection via different routes to the MF1 mouse fetus on day 15 of gestation and describe substantial gene delivery to the musculature. Highly efficient gene transfer to skeletal muscles, including the diaphragm and intercostal muscles, as well as to cardiac myocytes was observed and gene expression persisted for at least 15 months after administration of this integrating vector. These findings support the concept of in utero gene delivery for therapeutic and long-term prevention/correction of muscular dystrophies and pave the way for a future application in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Emery AEH . Duchenne Muscular Dystrophy. Oxford University Press: Oxford, 1993.
Simonds AK et al. Outcome of paediatric domiciliary mask ventilation in neuromuscular and skeletal disease. Eur Respir J 2000; 16: 476–481.
Turkel SB, Howell R, Iseri AL, Chui L . Ultrastructure of muscle in fetal Duchenne's dystrophy. Arch Pathol Lab Med 1981; 105: 414–418.
Yoshioka M, Yorifuji T, Mituyoshi I . Skewed X inactivation in manifesting carriers of Duchenne muscular dystrophy. Clin Genet 1998; 53: 102–107.
Wells DJ, Ferrer A, Wells KE . Immunological hurdles in the path to gene therapy for Duchenne muscular dystrophy. Expert Rev Mol Med 2002; 4: 1–23.
Chamberlain JS . Gene therapy of muscular dystrophy. Hum Mol Genet 2002; 11: 2355–2362.
Wells DJ, Wells KE . Gene transfer studies in animals: what do they really tell us about the prospects for gene therapy in DMD? Neuromuscul Disord 2002; 12 (Suppl 1): S11–S22.
Lipshutz GS et al. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther 2001; 3: 284–292.
Waddington SN et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood 2003; 101: 1359–1366.
Kafri T et al. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet 1997; 17: 314–317.
Naldini L, Verma IM . Lentiviral vectors. Adv Virus Res 2000; 55: 599–609.
MacKenzie TC et al. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther 2002; 6: 349–358.
Kobinger GP et al. Correction of the dystrophic phenotype by in vivo targeting of muscle progenitor cells. Hum Gene Ther 2003; 14: 1441–1449.
Waddington SN et al. Long-term transgene expression by administration of a lentivirus-based vector to the fetal circulation of immuno-competent mice. Gene Therapy 2003; 10: 1234–1240.
Yamada K, McCarty DM, Madden VJ, Walsh CE . Lentivirus vector purification using anion exchange HPLC leads to improved gene transfer. Biotechniques 2003; 34: 1074–1080.
O'Rourke JP et al. Analysis of gene transfer and expression in skeletal muscle using enhanced EIAV lentivirus vectors. Mol Ther 2003; 7: 632–639.
Seppen J, Barry SC, Harder B, Osborne WRA . Lentivirus administration to rat muscle provides efficient sustained expression of erythropoietin. Blood 2001; 98: 594–596.
Hu J, Dunbar CE . Update on haematopoietic stem cell gene transfer using non-human primate models. Curr Opin Mol Ther 2002; 4: 482–490.
Demaison C et al. High-level transduction and gene expression in haematopoietic repopulating cells using a human immunodeficiency [correction of immunodeficiency] virus type1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 2002; 13: 803–813.
Mitrophanous K et al. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Therapy 1999; 6: 1808–1818.
Olsen JC . Gene transfer vectors derived from equine infectious anemia virus. Gene Therapy 1998; 5: 1481–1487.
Stetor SR et al. Characterization of (+) strand initiation and termination sequences located at the center of the equine infectious anemia virus genome. Biochemistry 1999; 38: 3656–3667.
Donello JE, Loeb JE, Hope TJ . Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol 1998; 72: 5085–5092.
Martin-Rendon E et al. New methods to titrate EIAV-based lentiviral vectors. Mol Ther 2002; 5: 566–570.
Rohll JB et al. Design, production, safety, evaluation, and clinical applications of nonprimate lentiviral vectors. Methods Enzymol 2002; 346: 466–500.
Acknowledgements
LG is funded by a grant from the Muscular Dystrophy Association, USA. This work has been supported by an MRC Programme Grant. SW is salary funded by the Katherine Dormandy Trust.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gregory, L., Waddington, S., Holder, M. et al. Highly efficient EIAV-mediated in utero gene transfer and expression in the major muscle groups affected by Duchenne muscular dystrophy. Gene Ther 11, 1117–1125 (2004). https://doi.org/10.1038/sj.gt.3302268
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302268
Keywords
This article is cited by
-
Dystrophin Delivery to Muscles of mdx Mice Using Lentiviral Vectors Leads to Myogenic Progenitor Targeting and Stable Gene Expression
Molecular Therapy (2010)
-
The developmental stage determines the distribution and duration of gene expression after early intra-amniotic gene transfer using lentiviral vectors
Gene Therapy (2010)
-
Full-length dystrophin gene transfer to the mdx mouse in utero
Gene Therapy (2008)