Abstract
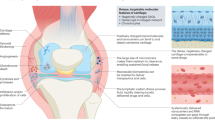
Rheumatoid arthritis is an autoimmune disease with intra-articular inflammation and synovial hyperplasia that results in progressive degradation of cartilage and bone, in severe cases it causes systemic complications. Recently, biological agents that suppress the activities of proinflammatory cytokines have shown efficacy as antiarthritic drugs, but require frequent administration. Thus, gene transfer approaches are being developed as an alternative approach for targeted, more efficient and sustained delivery of inhibitors of inflammatory cytokines as well as other therapeutic agents. Indeed, the efficacy of gene transfer for the treatment of arthritis has been demonstrated in mouse, rat, rabbit, and horse models of disease whereas the feasibility of the approach has been demonstrated in Phase I clinical trials. In this review, the current status of both preclinical and clinical arthritis gene therapy is presented. In addition, the advantages and disadvantages of different types of vectors, target cells and therapeutic genes being developed for the treatment of arthritis are summarized. Finally, the future directions of the rapidly developed field of arthritis gene therapy are outlined
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bresnihan B et al. Treatment of rheumatoid arthritis with recombinant human interelukin-1 receptor antagonist. Arthritis Rheum 1998; 41: 2196.
Jiang Y et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larson scores. Arthritis Rheum 2000; 43: 1001.
Moreland LW et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)–Fc fusion protein. N Engl J Med 1997; 337: 141.
Elliott MJ et al. Randomized double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor [alpha](cA2) versus placebo in rheumatoid arthritis. Lancet 1994; 244: 1105.
Robbins PD, Ghivizzani SC, Evans CH . Gene transfer to joints. Drug News Perspect 1997; 10: 283–292.
Evans CH, Robbins PD . Getting genes into human synovium. J Rheum 1997; 24: 2061–2063.
Gouze E, Ghivizzani SC, Robbins PD, Evans CH . Gene therapy for rheumatoid arthritis. Curr Rheumatol Rep 2001; 3: 142–146.
Nakajima A et al. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest 2001; 107: 1293–1301.
Chernajovsky Y et al. Inhibition of transfer of collagen-induced arthritis into SCID mice by ex vivo infection of spleen cells with retroviruses expressing soluble tumor necrosis factor receptor. Gene Therapy 1995; 2: 731–735.
Chernajovsky Y et al. Pathogenic lymphoid cells engineered to express TGF beta 1 ameliorate disease in a collagen-induced arthritis model. Gene Therapy 1997; 4: 553–559.
Kim SH et al. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol 2001; 166: 3499–3505.
Kim SH, Kim S, Oligino TJ, Robbins PD . Effective treatment of established mouse collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express FasL. Mol Ther 2002; 6: 584.
Morita Y et al. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen induced arthritis J Clin Invest 2001; 12: 1051–1061.
Saidenber-Kermanac'h N et al. Efficacy of interleukin-10 gene electrotransfer into skeletal muscle in mice with collagen induced arthritis. J Gene Med 2003; 5: 164–171.
Apparailly F et al. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol 1998; 160: 5213–5220..
Roessler BJ et al. Adenoviral mediated gene transfer to rabbit synovium in vivo. J Clin Invest 1993; 92: 1085–1092.
Ghivizzani SC et al. Direct adenoviral-mediated gene transfer of IL-1 and TNF-alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci USA 1998; 95: 4613–4618.
Zhang H et al. Amelioration of collagen-induced arthritis by CD95 (Apo-1/Fas)-ligand gene transfer. J Clin Invest 1997; 100: 1951.
Pan RY et al. Therapy and prevention of arthritis by recombinant adeno-associated virus vector in a rat arthritis model. Arthritis Rheum 2000; 43: 289–297.
Watanabe S et al. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synoviujm. Mol Ther 2000; 2: 147–152.
Gouze E et al. In vivo gene delivery to synovium by lentiviral vectors. Mol Ther 2002; 5: 397–404.
Ghivizanni SC, Oligino TJ, Glorioso JC, Robbins PD, Evans CH . Direct gene delivery strategies for the treatment of rheumatoid arthritis. Drug Discov Today 2001; 6: 259–267.
Ijimi K et al. Successful gene therapy via intraarticular injection of adenovirus vector containing CTLA4Ig in a murine model of type II collagen-induced arthritis. Hum Gene Ther 2001; 12: 1063–1077.
Woods JM et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J Immunol 2001; 166: 1214–1222.
Woods JM et al. Interleukin-13 gene therapy reduces inflammation, vascularization, and bony destruction in rat adjuvant-induced arthritis. Hum Gene Ther 2002; 13: 381–393.
Kim SH et al. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res 2000; 2: 293.
Lubberts E et al. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest 2000; 105: 1697–1710.
Lubberts E et al. Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. J Immunol 1999; 163: 4546–4556.
Whalen JD et al. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J Immunol 1999; 162: 3625.
Lechman ER et al. Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees. J Immunol 1999; 163: 2202.
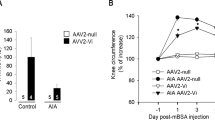
Miagkov AV et al. Endogenous regulation of a therapeutic transgene restores homeostasis in arthritic joints. J Clin Invest 2002; 109: 1223–1229.
Bendele A et al. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: comparison of efficacy in animal models with human clinical trial data. Arthritis Rheum 1999; 42: 498–506.
Otani K et al. Suppression of antigen-induced arthritis in rabbits by ex vivo gene therapy. J Immunol 1996; 156: 3558–3562.
Bandara G et al. Intraarticular expression of the interleukin-1 receptor antagonist protein by ex-vivo gene transfer. Proc Natl Acad Sci USA 1993; 90: 10764–10768.
Markarov SS et al. Suppression of experimental arthritis by gene transfer of the interluekin-1 receptor antagonists cDNA. Proc Natl Acad Sci USA 1996; 93: 402–406.
Bakker AC et al. Prevention of murine collagen induced arthritis in the knee and ipsilateral paw by local expression of human interluekin-1 receptor antagonist protein in the knee. Arthritis Rheum 1997; 40: 893–900.
Fernandes J et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol 1999; 154: 1159–1169.
Pelletier J-P et al. In vivo suppression of early experimental osteoarthritis by IL-1Ra using gene therapy. Arthritis Rheum 1997; 40: 1012–1019.
Frisbie DD et al. Treatment of experimental equine osteoarthritis by in vivo delivery of equine interleukin-1 receptor antagonist. Gene Therapy 2002; 9: 12–20.
Mi Z et al. Intra-articular effects of TGF-ß1 following adenoviral-mediated gene delivery. Arthritis Res., in press.
Song XY et al. Plasmid DNA encoding transforming growth factor-beta1 suppresses chronic disease in a streptococcal cell wall-induced arthritis model. J Clin Invest 1998; 101: 2615–2621.
Makarov SS et al. NF-kappa B as a target for anti-inflammatory gene therapy: suppression of inflammatory responses in monocytic and stromal cells by stable gene transfer of I kappa B alpha cDNA. Gene Therapy 1997; 4: 846–852.
Miagkov AV et al. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA 1998; 95: 13859–13864.
Tak PP et al. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 2001; 44: 1897–1907.
Tomita T et al. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor kappaB decoy oligodeoxynucleotide as a gene therapy. Arthritis Rheum. 1999; 42: 2532–2542.
Takayanagi H et al. Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts. J Clin Invest 1991; 104: 137–146.
Shouda T et al. Induction of cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis J Clin Invest 2001; 108: 1781–1788.
Sant SM et al. Molecular lysis of synovial lining cells by in vivo herpes simplex virus-thymidine kinase gene transfer. Hum Gene Ther 1998; 9: 2735–2743.
Goossens PH et al. Feasibility of adenovirus-mediated nonsurgical synovectomy in collagen-induced arthritis-affected rhesus monkeys. Hum Gene Ther 1999; 10: 1139–1149.
Yao Q et al. Adenoviral mediated delivery of Fas ligand to rheumatoid synovium results in extensive apoptosis in the synovial lining. J Gene Med 2000; 2: 210–219.
Okamoto K et al. Induction of apoptosis in the rheumatoid synovium by Fas ligand gene transfer. Gene Therapy 1998; 5: 331–338.
Yao Q et al. TRAIL induced apoptosis in the rabbit synovium. Gene Therapy, in press.
Yao Q et al. Gene transfer of p53 to arthritis joints stimulates synovial apoptosis and inhibits inflammation. Mol Ther 2001; 3: 901–910.
Taniguchi K et al. Induction of the p16IKNK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat Med 1999; 5: 760–767.
Nasu K et al. Adenoviral transfer of cyclin dependent kinase inhibitor genes suppresses collagen-induced arthritis in mice. J Immunol 2000; 165: 7246–7252.
Yin G et al. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol Ther 2002; 5: 547–554.
Kim JM et al. Angiostatin gene transfer as an effective treatment strategy in murine collagen induced arthritis. Arthritis Rheum 2002; 46: 793–801.
Varley AW, Geiszler SM, Gaynor RB, Munford RS . A two component expression system that responds to inflammatory stimuli in vivo. Nat Biotechnol 1997; 15: 1002–1006.
Bakker AC et al. C3-Tat/HIV-regulated intraarticular human interleukin-1 receptor antagonist gene therapy results in efficient inhibition of collagen-induced arthritis superior to cytomegalovirus-regulated expression of the same transgene. Arthritis Rheum 2002; 46: 1661–1670.
Gould DJ et al. A novel doxycycline inducible autoregulatory plasmid which displays “on”/”off” regulation suited to gene therapy applications. Gene Therapy 2000; 7: 2061–2070.
Gould D, Bright C, Chernajovsky Y . Inhibition of collagen induced arthritis with dTNF-R expressed from a doxycycline regulatable plasmid. Rheumatology 2002; 41: 29.
Dreja H, Annenkov A, Chernajovsky Y . Soluble complement receptor 1 (CD35) delivered by retrovirally infected syngeneic cells or by naked DNA injection prevents the progression of collagen-induced arthritis. Arthritis Rheum 2000; 43: 1698–1709.
Misaki Y et al. Antigen-specific T cells transduced with interleukin-10 ameliorate experimentally induced arthritis without impairing the systemic immune response to the antigen. Nihon Rinsho Meneki Gakkai Kaishi 2000; 23: 538–541.
Chernajovsky Y, Feldmann M, Maini RN . Gene therapy of rheumatoid arthritis via cytokine regulation: future perspectives. Br Med Bull 1995; 51: 503–516.
Mageed RA et al. Prevention of collagen-induced arthritis by gene delivery of soluble p75 tumour necrosis factor receptor. Gene Therapy 1998; 5: 1584–1592.
Williams RO, Feldmann M, Maini RN . Anti-TNF ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA 1992; 89: 9784–9788.
Annenkov AE et al. Loss of original antigenic specificity in T cell hybridomas transduced with a chimeric receptor containing single-chain Fv of an anti-collagen antibody and Fc epsilonRI-signaling gamma subunit. J Immunol 1998; 161: 6604–6613.
Annenkov A, Chernajovsky Y . Engineering mouse T lymphocytes specific to type II collagen by transduction with a chimeric receptor consisting of a single chain Fv and TCR zeta. Gene Therapy 2000; 7: 714–722.
Rabinovich GA et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999; 190: 385–398.
Bessis N et al. Syngeneic fibroblasts transfected with a plasmid encoding interleukin-4 as non-viral vectors for anti-inflammatory gene therapy in collagen- induced arthritis. J Gene Med 2002; 4: 300–307.
Rosloniec EF et al. Vaccination with a recombinant V alpha domain of a TCR prevents the development of collagen-induced arthritis. J Immunol 1995; 155: 4504–4511.
Quattrocchi E, Dallman MJ and Feldmann M . Adenovirus-mediated gene transfer of CTLA-4Ig fusion protein in the suppression of experimental autoimmune arthritis. Arthritis Rheum 2000; 43: 1688–1697.
Guery L et al. Collagen II-pulsed antigen-presenting cells genetically modified to secrete IL-4 down-regulate collagen-induced arthritis. Gene Therapy 2001; 8: 1855–1862.
Bessis N et al. Modulation of proinflammatory cytokine production in tumour necrosis factor-alpha (TNF-alpha)-transgenic mice by treatment with cells engineered to secrete IL-4, IL-10 or IL-13. Clin Exp Immunol 1998; 111: 391–396.
Bessis N et al. Encapsulation in hollow fibres of xenogeneic cells engineered to secrete IL-4 or IL-13 ameliorates murine collagen-induced arthritis (CIA). Clin Exp Immunol. 1999; 117: 376–382.
Triantaphyllopoulos KA et al. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum 1999; 42: 90–99.
Neve R et al. Expression of an efficient small molecular weight tumour necrosis factor/lymphotoxin antagonist. Cytokine 1996; 8: 365–370.
Chernajovsky Y . Systemic gene therapy for arthritis. Drugs Today 1999; 35: 361–377.
Freudenberg MA et al. A murine, IL-12-independent pathway of IFN-gamma induction by Gram-negative bacteria based on STAT4 activation by Type I IFN and IL-18 signaling. J Immunol 2002; 169: 665–1668.
Farrar JD et al. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat Immunol 2000; 1: 65–69.
Takayanagi H, Kim S, Taniguchi T . Signaling crosstalk between RANK-L and interferons in osteoclastogenesis. Arthritis Res 2002; 4: S227–S232.
Fellowes R et al. Amelioration of established collagen induced arthritis by systemic IL- 10 gene delivery. Gene Therapy 2000; 7: 967–977.
Bessis N et al. The type II decoy receptor of IL-1 inhibits murine collagen-induced arthritis. Eur J Immunol, 2000; 30: 867–875.
Tomita T et al. Transcription factor decoy for NFkappaB inhibits cytokine and adhesion molecule expressions in synovial cells derived from rheumatoid arthritis. Rheumatology (Oxford) 2000; 39: 749–757.
Iyama S et al. Treatment of murine collagen-induced arthritis by ex vivo extracellular superoxide dismutase gene transfer. Arthritis Rheum 2001; 44: 2160–2167.
Dai L et al. Amelioration of antigen-induced arthritis in rats by transfer of extracellular superoxide dismutase and catalase genes. Gene Therapy, in press.
Apparailly F et al. Paradoxical effects of tissue inhibitor of metalloproteinases 1 gene transfer in collagen-induced arthritis. Arthritis Rheum 2001; 44: 1444–1454.
Whalen JD et al. Viral IL-10 gene transfer inhibits DTH responses to soluble antigens: evidence for involvement of genetically modified dendritic cells and macrophages. Mol Ther 2001; 4: 543–550.
Kim SH et al. Intra-articular, ex vivo delivery of the IL-1 receptor antagonist and soluble TNF-alpha receptor confers a therapeutic effect in both the treated and untreated contralateral joints in a rabbit antigen-induced arthritis model. Mol Ther 2002; 6: 591–600.
Lechman ER et al. The contralateral effect conferred by intra-articular adenovirus mediated gene transfer of vIL-10 is antigen specific. Gene Therapy, in press.
Evans CH et al. Clinical trials in the gene therapy of arthritis. Clin Ortho 2000; 379: 300–307.
Evans CH et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther 1996; 7: 1261–1280.
Feldmann M, Brennan FM, Abney ER, Hales A, Chernajovsky Y, Katsikis P, Corcoran A, Haworth C, Cope A, Gibbons D, Chu CQ, Field M, Deleuran B, Williams RO, Maini RN . Are imbalances in cytokine function of importance in the pathogenesis of RA? In: Davies E, Dingle JD (eds.), Immunopharmacology of Joints and Connective Tissue. Academic Press: New York, 1994, pp. 119–128.
Maini RN, Taylor PC . Anti-cytokine therapy for rheumatoid arthritis. Annu Rev Med 2000; 51: 207–229.
Firestein GS . Novel therapeutic strategies involving animals, arthritis, and apoptosis. Curr Opin Rheumatol 1998; 10: 236–241.
Muller-Ladner U et al. Gene transfer of cytokine inhibitors into human synovial fibroblasts in the SCID mouse model. Arthritis Rheum 1999; 42: 490–497.
Firestein GS et al. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA 1997; 94: 10895–10900.
Tak PP et al. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today 2000; 21: 78–82.
Chernajovsky Y, Winyard PG, Kabouridis PS . Advances in understanding the genetic basis of rheumatoid arthritis: implications for therapy. Am J Pharmacogenomics. 2003; 2: 223–234.
Muller-Ladner U, Nishioka K . p53 in rheumatoid arthritis: friend or foe? Arthritis Res 2000; 2: 175–178.
Adams G, Dreja H, Chernajovsky Y . Targeting cytokines to sites of inflammation. Rheumatology 2002; 41(S1): 29.
Gofur Y, Chernajovsky Y . The development of immunocytokines for the treatment of rheumatoid arthritis. Rheumatology 2002; 41(S1): 28.
Chernajovsky Y et al. Gene therapy for rheumatoid arthritis. Theoretical considerations. Drugs Aging 1998; 12: 29–41.
Robbins PD, Evans CH, Chernajovsky Y . Gene therapy for rheumatoid arthritis. Springer Semin Immunopathol 1998; 20: 197–209.
Kim KN et al. Viral IL-10 and soluble TNF receptor act synergistically to inhibit collagen-induced arthritis following adenovirus-mediated gene transfer. J Immunol 2000; 164: 1576–1581.
Müller-Ladner U et al. Double and triple gene transfer in arthritis. Arthritis Res 2001; 3S 1: a5.
Delgado M et al. Vasoactive intestinal peptide prevents experimental arthritis downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001; 7: 563–568.
Lubberts E et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol 2001; 167: 004–1013.
Waldmann TA, Tagaya Y . The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 1999; 17: 19–49.
Jorgensen C et al. Stem cells for repair of cartilage and bone: the next challenge in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis 2001; 60: 305–309.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robbins, P., Evans, C. & Chernajovsky, Y. Gene therapy for arthritis. Gene Ther 10, 902–911 (2003). https://doi.org/10.1038/sj.gt.3302040
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302040
This article is cited by
-
Lactobacillus acidophilus Protected Organs in Experimental Arthritis by Regulating the Pro-inflammatory Cytokines
Indian Journal of Clinical Biochemistry (2014)
-
La terapia genica nella riparazione cartilaginea
Archivio di Ortopedia e Reumatologia (2009)
-
Technology Insight: adult mesenchymal stem cells for osteoarthritis therapy
Nature Clinical Practice Rheumatology (2008)
-
Local gene transfer to calcified tissue cells using prolonged infusion of a lentiviral vector
Gene Therapy (2006)
-
Large animal models and gene therapy
European Journal of Human Genetics (2006)