Abstract
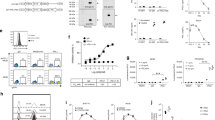
Tumor cells engineered by gene transduction to be MHC Class II+/Ii- are novel APCs capable of presenting endogenous tumor antigen epitopes to activate T helper cells. The MHC Class II+/Ii- tumor cell phenotype is created by transfecting genes for either CIITA or IFN-γ, and inhibiting induced Ii mRNA by an Ii reverse gene construct (Ii-RGC). Adenoviral vectors are preferred for the delivery of such genes because of high infection efficiency and ubiquity of the adenoviral receptor on many cell types and tumors. Here we show that at 5 MOI (multiplicity of infection), recombinant adenoviruses with CIITA or IFN-γ genes converted virtually all MC-38 colon adenocarcinoma cells and Renca renal carcinoma cells in culture to MHC Class II+/Ii+ cells. A single recombinant adenovirus with both genes for IFN-γ and Ii-RGC (rAV/IFN-γ/Ii-RGC) efficiently induced the MHC Class II+/Ii- phenotype. Injection of tumor nodules with rAV/Ii-RGC and rAV/CIITA/IFN-γ combined with a suboptimal dose of rAV/IL-2 induced a potent antitumor immune response. The methods are adaptable for producing enhanced genetic vaccines, attenuated virus vaccines (eg, vaccinia), and ex vivo cell-based vaccines (dendritic and tumor cells).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pardoll DM, Topalian SL . The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 1998; 10: 588–594.
Wang RF . The role of MHC Class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol 2001; 22: 269–276.
Ostrand-Rosenberg S et al. Cell-based vaccines for the stimulation of immunity to metastatic cancers. Immunol Rev 1999; 170: 101–114.
Clements VK, Baskar S, Armstrong TD, Ostrand-Rosenberg S . Invariant chain alters the malignant phenotype of MHC Class II+ tumor cells. J Immunol 1992; 149: 2391–2396.
Armstrong TD et al. Major histocompatibility complex Class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA 1997; 94: 6886–6891.
Chang CH, Flavell RA . Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med 1995; 181: 765–767.
Boss JM . Regulation of transcription of MHC Class II genes. Curr Opin Immunol 1997; 9: 107–113.
Qiu G, Goodchild J, Humphreys RE, Xu M . Cancer immunotherapy by antisense suppression of Ii protein in MHC-Class-II-positive tumor cells. Cancer Immunol Immunother 1999; 48: 499–506.
Lu L et al. Tumor immunotherapy by converting tumor cells to MHC Class II-positive, Ii protein-negative phenotype (submitted for publication).
Berkner KL . Expression of heterogonous sequences in adeno-viral vectors. Curr Top Microbiol Immunol 1992; 158: 39–66.
Vorburger SA, Hunt KK . Adenoviral gene therapy. Oncologist 2002; 7: 46–59.
Danthinne X, Imperiale MJ . Production of first generation adenovirus vectors: a review. Gene Therapy 2000; 7: 1707–1714.
Kovesdi I, Brough DE, Bruder JT, Wickham TJ . Adenoviral vectors for gene transfer. Curr Opin Biotechnol 1997; 8: 583–589.
Graham FL, Prevec L . Manipulation of adenovirus vectors. In: Murray EJ (ed). Methods in Molecular Biology. Humana: Clifton, NJ, 1991, Vol 7, pp 4109–4128.
Trapnell BC, Gorziglia M . Gene therapy using adenoviral vectors. Curr Opin Biotechnol 1994; 5: 617–625.
Lotze MT, Kost TA . Viruses as gene delivery vectors: application to gene function, target validation, and assay development. Cancer Gene Ther 2002; 9: 692–699.
Aoki T et al. Inhibition of autocrine fibroblast growth factor signaling by the adenovirus-mediated expression of an antisense transgene or a dominant negative receptor in human glioma cells in vitro. Int J Oncol 2002; 21: 629–636.
Im SA et al. Inhibition of breast cancer growth in vivo by antiangiogenesis gene therapy with adenovirus-mediated antisense-VEGF. Br J Cancer 2001; 84: 1252–1257.
Inoue K et al. Gene therapy of human bladder cancer with adenovirus-mediated antisense basic fibroblast growth factor. Clin Cancer Res 2000; 6: 4422–4431.
Fan Z et al. Adenovirus-mediated antisense ATM gene transfer sensitizes prostate cancer cells to radiation. Cancer Gene Ther 2000; 7: 1307–1314.
Wang X et al. Episomal segregation of the adenovirus enhancer sequence by conditional genome rearrangement abrogates late viral gene expression. J Virol 2000; 74: 11296–11303.
Bertolino P, Rabourdin-Combe C . The MHC Class II-associated invariant chain: a molecule with multiple roles in MHC Class II biosynthesis and antigen presentation to CD4+ T cells. Crit Rev Immunol 1996; 16: 359–379.
Ting JP, Trowsdale J . Genetic control of MHC Class II expression. Cell 2002; 109(Suppl): S21–S33.
Barna BP et al. Therapeutic effects of a synthetic peptide of C-reactive protein in pre-clinical tumor models. Cancer Immunol Immunother 1993; 36: 171–176.
Dezso B et al. The mechanism of local tumor irradiation combined with interleukin 2 therapy in murine renal carcinoma: histological evaluation of pulmonary metastases. Clin Cancer Res 1996; 2: 1543–1552.
Koch N, Koch S, Hammerling GJ . Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature 1982; 299: 644–645.
Long EO et al. Efficient cDNA expression vectors for stable and transient expression of HLA-DR in transfected fibroblast and lymphoid cells. Hum Immunol 1991; 31: 229–235.
Chen PW, Ananthaswamy HN . Rejection of K1735 murine melanoma in syngeneic hosts requires expression of MHC Class I antigens and either Class II antigens or IL-2. J Immunol 1993; 151: 244–255.
Zhou H, Glimcher LH . Human MHC Class II gene transcription directed by the carboxyl terminus of CIITA, one of the defective genes in type II MHC combined immune deficiency. Immunity 1995; 2: 545–553.
Slos P et al. Immunotherapy of established tumors in mice by intratumoral injection of an adenovirus vector harboring the human IL-2 cDNA: induction of CD8(+) T-cell immunity and NK activity. Cancer Gene Ther 2001; 8: 321–332.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hillman, G., Kallinteris, N., Li, J. et al. Generating MHC Class II+/Ii- phenotype after adenoviral delivery of both an expressible gene for MHC Class II inducer and an antisense Ii-RNA construct in tumor cells. Gene Ther 10, 1512–1518 (2003). https://doi.org/10.1038/sj.gt.3302027
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302027
Keywords
This article is cited by
-
Regulation of MHC class II gene expression by the class II transactivator
Nature Reviews Immunology (2005)