Abstract
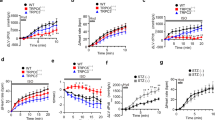
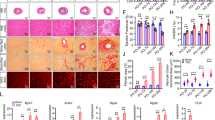
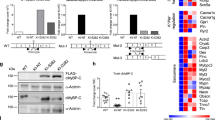
Myocardial overexpression of the C-terminus of β-adrenergic receptor kinase (βARKct) has been shown to result in a positive inotropic effect or an improvement of survival in heart failure. However, it is not clear whether this beneficial effect is mainly because of dominant-negative inhibition of βARK1, and a consecutive resensitization of β-adrenergic receptors (βAR), or rather due to inhibition of other Gβγ-mediated effects. In this study, we tested whether overexpression of N-terminally truncated phosducin (nt-del-phosducin), another Gβγ-binding protein that does not resensitize βARs owing to simultaneous inhibition of GDP release from Gα subunits, shows the same effects as βARKct. Adenoviral gene transfer was used to express nt-del-phosducin and βARKct in isolated ventricular cardiomyocytes and in myocardium of rabbits, which suffered from heart failure because of rapid ventricular pacing. βAR-stimulated cAMP formation was increased by βARKct, but not by nt-del-phosducin, whereas both proteins inhibited Gβγ-mediated effects. Both transgenes also increased contractility of normal and failing isolated cardiomyocytes and improved contractility in rabbits with heart failure after gene transfer in vivo. In conclusion, overexpression of nt-del-phosducin enhances the contractility of cardiomyocytes to the same extent as βARKct, suggesting that the effects of βARKct might be owing to inhibition of Gβγ rather than to βAR resensitization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ungerer M et al. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in failing human heart. Circulation 1993; 87: 454–463.
Lohse MJ . Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta 1993; 1179: 171–188.
Lefkowitz RJ . G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 1998; 273: 18677–18680.
Pitcher JA et al. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science 1992; 257: 1264–1267.
Akhter SA et al. Restoration of adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proc Natl Acad Sci USA 1997; 94: 12100–12105.
White DC et al. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci USA 2000; 97: 5428–5433.
Shah AS et al. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation 2001; 103: 1311–1316.
Rockman HA et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA 1998; 95: 7000–7005.
Freeman K et al. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest 2001; 107: 967–974.
Harding VB et al. Cardiac βARK1 inhibition prolongs survival and augments β-blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci USA 2001; 98: 5809–5814.
Hamm HE . The many faces of G protein signaling. J Biol Chem 1998; 273: 669–672.
Koch WJ et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a βARK inhibitor. Science 1995; 268: 1350–1353.
Gaudet R, Bohm A, Sigler PB . Crystal structure at 2.4 angstroms resolution of the complex of transducin beta gamma and its regulator, phosducin. Cell 1996; 87: 577–588.
Bauer PH et al. Phosducin is a protein kinase A-regulated G protein regulator. Nature 1992; 358: 73–76.
Hekman M, Bauer PH, Söhlemann P, Lohse MJ . Phosducin inhibits receptor phosphorylation by the beta-adrenergic receptor kinase in a PKA-regulated manner. FEBS Lett 1994; 343: 120–124.
Müller S et al. Interactions of phosducin with defined Gβγ subunits. J Biol Chem 1996; 271: 11781–11786.
Blüml K et al. A small region in phosducin inhibits G-protein βy subunit function. J Biol Chem 1997; 16: 4908–4915.
Schulz K et al. Expression of phosducin in a phosducin-negative cell line reveals functions of a Gβγ-binding protein. J Biol Chem 1996; 271: 22546–22551.
Laugwitz KL et al. Blocking caspase-activated apoptosis improves contractility in failing myocardium. Hum Gene Ther 2001; 12: 2051–2063.
Korzick DH et al. Transgenic manipulation of beta-adrenergic receptor kinase modifies cardiac myocyte contraction to norepinephrine. Am J Physiol 1997; 272: H590–H596.
Clapham DE, Neer EJ . New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature 1993; 365: 403–436.
Luttrell LM et al. β-arrestin-dependent formation of β2-adrenergic receptor – src protein kinase complexes. Science 1999; 283: 655–661.
Naga-Prasad SV et al. G protein-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J Biol Chem 2000; 275: 4693–4698.
Yoshida T et al. The phosphorylation state of phosducin determines its ability to block transducin subunit interactions and inhibit transducin binding to activated rhodopsin. J Biol Chem 1994; 269: 24050–24057.
Thulin CD et al. Modulation of the G protein regulator phosducin by Ca2+/calmodulin-dependent protein kinase II phosphorylation and 14-3-3 protein binding. J Biol Chem 2001; 276: 23805–23815.
He TC et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Weig HJ et al. Enhanced cardiac contractility after gene transfer of V2 vasopressin receptors in vivo by ultrasound-guided injection or trans-coronary delivery. Circulation 2000; 101: 1578–1585.
Laugwitz KL et al. Gene transfer of heterologous G protein-coupled receptors to cardiomyocytes: differential effects on contractility. Circ Res 2001; 88: 688–695.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976; 72: 248–254.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Z., Laugwitz, KL., Pinkernell, K. et al. Effects of two Gβγ-binding proteins – N-terminally truncated phosducin and β-adrenergic receptor kinase C terminus (βARKct) – in heart failure. Gene Ther 10, 1354–1361 (2003). https://doi.org/10.1038/sj.gt.3301995
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301995
This article is cited by
-
Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor
Basic Research in Cardiology (2017)
-
Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies
Journal of Translational Medicine (2013)
-
Targeting GRK2 by gene therapy for heart failure: benefits above β-blockade
Gene Therapy (2012)
-
The physiological roles of phosducin: from retinal function to stress-dependent hypertension
Cellular and Molecular Life Sciences (2011)
-
Novel pharmacotherapies to abrogate postinfarction ventricular remodeling
Nature Reviews Cardiology (2009)