Abstract
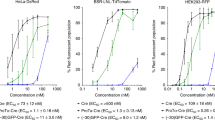
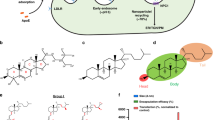
Presence of endosome-disrupting multiple histidine functionalities in the molecular architecture of cationic polymers, such as polylysine, has previously been demonstrated to significantly enhance their in vitro gene delivery efficiencies. Towards harnessing improved transfection property through covalent grafting of endosome-disrupting single histidine functionality in the molecular structure of cationic lipids, herein, we report on the design, the synthesis and the transfection efficiency of two novel nonglycerol-based histidylated cationic amphiphiles. We found that L-histidine-(N,N-di-n-hexadecylamine)ethylamide (lipid 1) and L-histidine-(N,N-di-n-hexadecylamine,-N-methyl)ethylamide (lipid 2) in combination with cholesterol gave efficient transfections into various cell lines. The transfection efficiency of Chol/lipid 1 lipoplexes into HepG2 cells was two order of magnitude higher than that of FuGENETM6 and DC-Chol lipoplexes, whereas it was similar into A549, 293T7 and HeLa cells. A better efficiency was obtained with Chol/lipid 2 lipoplexes when using the cytosolic luciferase expression vector (pT7Luc) under the control of the bacterial T7 promoter. Membrane fusion activity measurements using fluorescence resonance energy transfer (FRET) technique showed that the histidine head-groups of Chol/lipid 1 liposomes mediated membrane fusion in the pH range 5–7. In addition, the transgene expression results using the T7Luc expression vector convincingly support the endosome-disrupting role of the presently described mono-histidylated cationic transfection lipids and the release of DNA into the cytosol. We conclude that covalent grafting of a single histidine amino acid residue to suitable twin-chain hydrophobic compounds is able to impart remarkable transfection properties on the resulting mono-histidylated cationic amphiphile, presumably via the endosome-disrupting characteristics of the histidine functionalities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mountain A . Gene therapy: the first decade. Trends Biotechnol 2000; 18: 119–128.
Verma IM, Somina M . Gene therapy – promises, problems and prospects. Nature 1997; 389: 239–242.
Felgner PL et al. Lipofection: a highly efficient, lipid-mediated DNA transfection procedure. Proc Natl Acad Sci USA 1987; 84: 7413–7417.
Gao X, Huang L . Cationic liposome-mediated gene transfer. Gene Ther 1995; 2: 710–722.
Guenin E et al. Cationic phosphonolipids containing quaternary phosphonium and arsonium groups for DNA transfection with good efficiency and low cellular toxicity. Angew Chem Int Ed Engl 2000; 39: 629–631.
Floch V et al. Cation substitution in cationic phosphonolipids: a new concept to improve transfection activity and decrease cellular toxicity. J Med Chem 2000; 43: 4617–4628.
Floch V et al. Systemic administration of cationic phosphonolipids/DNA complexes and the relationship between formulation and lung transfection efficiency. Biochim Biophys Acta 2000; 1464: 95–103.
Pitard B et al. Sterically stabilized BGTC-based lipoplexes: structural features and gene transfection into the mouse airways in vivo. J Gene Med 2001; 3: 478–487.
Oudrhiri N et al. Gene transfer by guanidinium-cholesterol cationic lipids into airway epithelial cells in vitro and in vivo. Proc Natl Acad Sci USA 1997; 94: 1651–1656.
Tan F, Hughes JA . Introduction of a disulfide bond into a cationic lipid enhances transgene expression of plasmid DNA. Biochem Biophys Res Commun 1998; 242: 141–145.
Banerjee R et al. Design, synthesis and transfection biology of novel cationic glycolipids for use in liposomal gene delivery. J Med Chem 2001; 44: 4176–4185.
Ayyagari AA et al. Novel non-glycerol based cytofectins with lactic acid derived head groups. Biochem Biophys Res Commun 2001; 289: 1057–1062.
Sunil Singh R et al. Anchor-dependency for non-glycerol based cationic lipofectins: mixed bag of regular and anomalous transfection profiles. Chem Eur J 2002; 8: 900–909.
Sri Lakshmi GV et al. Anchor-dependent Lipofection with non-glycerol based cytofectins containing single 2-hydroxyethyl head groups. Biochim Biophys Acta 2002; 1559: 87–95.
Banerjee R et al. A novel series of non-glycerol based cationic transfection lipids for use in liposomal gene delivery. J Med Chem 1999; 42: 4292–4299.
Bally MB et al. Biological barriers to cellular delivery of lipid-based DNA carriers. Adv Drug Deliv Rev 1999; 38: 291–315.
Zabner J et al. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem 1995; 270: 18997–19007.
Friend DS, Papahadjopoulos D, Debs RJ . Endocytosis and Intracellular processing accompanying transfection mediated by cationic liposomes. Biochim Biophys Acta 1996; 1278: 41–50.
Xu Y, Szoka Jr FC . Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996; 35: 5616–5623.
Pichon C, Gonçalves C, Midoux P . Histidine-rich peptides and polymers for nucleic acids delivery. Adv Drug Deliv Rev 2001; 53: 75–94.
Midoux P, Monsigny M . Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjugate Chem 1999; 10: 406–411.
Benns JM et al. pH-sensitive cationic polymer gene delivery vehicle:N-Ac-poly(L-histidine)-graft-poly(L-lysine) comb shaped polymer. Bioconjugate Chem 2000; 11: 637–645.
Chen Q-R, Zhang L, Stass SA, Mixon AJ . Co-polymer of histidine and lysine markedly enhances transfection efficiency of liposomes. Gene Ther 2000; 7: 1698–1705.
Chen Q-R, Zhang L, Luther PW, Mixson AJ . Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res 2002; 30: 1338–1345.
Brisson M et al. Subcellular trafficking of the cytoplasmic expression system. Hum Gene Ther 1999; 10: 2601–2613.
Gao X, Huang L . Cytoplasmic expression of a reporter gene by co-delivery of T7 RNA polymerase and T7 promoter sequence with cationic liposomes. Nucleic Acids Res 1993; 21: 2867–2872.
Gao X, Jaffurs D, Robbins PD, Huang L . A sustained cytoplasmic transgene expression system delivered by cationic liposomes. Biochem Biophys Res Commun 1994; 200: 1201–1206.
Deng H, Wolff JA . Self-amplifying Expression from the T7 Promoter in 3T3 mouse fibroblasts. Gene 1994; 143: 245–249.
Li S, Brisson M, He Y, Huang L . Delivery of a PCR amplified DNA fragment into cells: a model for using synthetic genes for gene therapy. Gene Ther 1997; 4: 449–454.
Struck DK, Hoekstra D, Pagano RE . Use of resonance energy transfer to monitor membrane fusion. Biochemistry 1981; 20: 4093–4099.
Heyes JA, Duvaz DN, Cooper RG, Springer CJ . Synthesis of novel cationic lipids: effect of structural modification on the efficiency of gene transfer. J Med Chem 2001; 45: 99–114.
Chen TR . In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoeschst 33258 stain. Exp Cell Res 1977; 104: 255–262.
De Wet JR et al. Firefly luciferase gene structure and expression in mammalian cells. Mol Cell Biol 1987; 7: 725–737.
Smith PK et al. Measurement of protein using bicinchononic acid. Anal Biochem 1985; 150: 76–85.
Hill HD, Straka JG . Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem 1988; 170: 203–208.
Acknowledgements
We express our gratitude to Dr JP Behr (Strasbourg, France) for initiating this Indo-French collaboration. We thank warmly Drs L Huang and M Brisson (University of Pittsburgh, Pittsburgh, PA, USA) for 293T7 cells and pT7Luc, and Miss Laëtitia Million for her help in FRET measurements. This work was supported by grants from Agence Nationale de Recherche sur le Sida (ANRS) and Association pour la Recherche sur le Cancer (ARC). AC and VVK gratefully acknowledge the financial support received from the Department of Biotechnology, Government of India, New Delhi. PM is Research Director at INSERM. CP is Professor Assistant at the University of Orléans.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, V., Pichon, C., Refregiers, M. et al. Single histidine residue in head-group region is sufficient to impart remarkable gene transfection properties to cationic lipids: evidence for histidine-mediated membrane fusion at acidic pH. Gene Ther 10, 1206–1215 (2003). https://doi.org/10.1038/sj.gt.3301979
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301979
Keywords
This article is cited by
-
An assessment of the transport mechanism and intraneuronal stability of l-carnosine
Amino Acids (2022)
-
Biological activities of histidine-rich peptides; merging biotechnology and nanomedicine
Microbial Cell Factories (2011)
-
Synthesis of novel porphyrin-based lipids and their antibacterial activity
Medicinal Chemistry Research (2011)
-
Nature as a source of inspiration for cationic lipid synthesis
Genetica (2010)
-
Preparation and gene delivery of alkaline amino acids-based cationic liposomes
Archives of Pharmacal Research (2008)