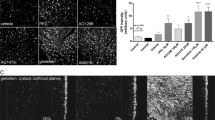
Abstract
Although lentivirus vectors hold promise for ocular gene therapy, they also have potential safety issues, particularly in the case of the current human immunodeficiency virus-based vectors. We recently developed a novel lentivirus vector derived from the nonpathogenic simian immunodeficiency virus from African green monkeys (SIVagm) to minimize these potentials. In this preclinical study, we evaluated whether SIV vector could be efficiently and safely applicable to retinal gene transfer by assessing the transgene expression, retinal function and histology over a 1-year period following subretinal injection in adult rats. The functional assessment via electroretinogram after both titers of SIV-lacZ (2.5 × 107 or 2.5 × 108 transducing units/ml) injection revealed both the dark and light adaptations to soon be impaired, in a dose-dependent manner, after a buffer injection as well, and all of them recovered to the control range by day 30. In both titers tested, the retinas demonstrated a frequent transgene expression mainly in the retinal pigment epithelium; however, the other retinal cells rarely expressed the transgene. Retinas exposed to a low titer virus showed no significant inflammatory reaction throughout the observation period, and also maintained the transgene expression over a 1-year period. In the retinas exposed to a high titer virus, however, mononuclear cell infiltration persisted in the subretinal area, and the retina that corresponded to the injected area finally underwent degeneration by around day 90. No retinal neoplastic lesions could be found in any animals over the 1-year period. We thus propose that SIV-mediated stable gene transfer might be useful for ocular gene transfer; however, more attention should be paid to avoiding complications when administering high titer lentivirus to the retina.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pagon RA . Retinitis pigmentosa. Surv Ophthalmol 1988; 33: 137–177.
Wong P . Apoptosis, retinitis pigmentosa, and degeneration. Biochem Cell Biol 1994; 72: 489–498.
Dryja TP et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 1990; 343: 364–366.
Kajiwara K et al. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature 1991; 354: 480–483.
Cotran PR et al. Genetic analysis of patients with retinitis pigmentosa using a cloned cDNA probe for the human gamma subunit of cyclic GMP phosphodiesterase. Exp Eye Res 1991; 53: 557–564.
Bennett J et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med 1996; 2: 649–654.
Cayouette M, Gravel C . Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther 1997; 8: 423–430.
Bennett J et al. Adenovirus-mediated delivery of rhodopsin-promoted bcl-2 results in a delay in photoreceptor cell death in the rd/rd mouse. Gene Therapy 1998; 5: 1156–1164.
Sakamoto T et al. Retinal functional change caused by adenoviral vector-mediated transfection of LacZ gene. Hum Gene Ther 1998; 9: 789–799.
Ali RR et al. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet 1996; 5: 591–594.
Flannery JG et al. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA 1997; 94: 6916–6921.
Bennett J et al. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Invest Ophthalmol Vis Sci 1997; 38: 2857–2863.
Lewin AS et al. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med 1998; 4: 967–971.
Bennett J et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci USA 1999; 96: 9920–9925.
Miyoshi H et al. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA 1997; 94: 10319–10323.
Takahashi M et al. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol 1999; 73: 7812–7816.
Ali RR et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet 2000; 25: 306–310.
Naldini L et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Nakajima T et al. Development of novel simian immunodeficiency virus vectors carrying a dual gene expression system. Hum Gene Ther 2000; 11: 1863–1874.
Auricchio A et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 2001; 10: 3075–3081.
Bainbridge JW et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Therapy 2001; 8: 1665–1668.
Duisit G et al. Five recombinant simian immunodeficiency virus pseudotypes lead to exclusive transduction of retinal pigmented epithelium in rat. Mol Ther 2002; 6: 446–454.
Sakamoto T et al. A vitrectomy improves the transfection efficiency of adenoviral vector-mediated gene transfer to Muller cells. Gene Therapy 1998; 5: 1088–1097.
Reichel MB et al. Immune responses limit adenovirally mediated gene expression in the adult mouse eye. Gene Therapy 1998; 5: 1038–1046.
Van Ginkel FW et al. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum Gene Ther 1995; 6: 895–903.
Van Ginkel FW et al. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol 1997; 159: 685–693.
Stripecke R et al. Immune response to green fluorescent protein: implications for gene therapy. Gene Therapy 1999; 6: 1305–1312.
Doi K et al. Lentiviral transduction of green fluorescent protein in retinal epithelium: evidence of rejection. Vision Res 2002; 42: 551–558.
Johnson PR et al. Molecular clones of SIVsm and SIVagm: experimental infection of macaques and African green monkeys. J Med Primatol 1990; 19: 279–286.
Ikeda Y et al. Recombinant sendai virus-mediated gene transfer into the retinal tissue of adult rats: efficient gene transfer by brief exposure. Exp Eye Res 2002; 75: 39–48.
Goto Y et al. Functional abnormalities in transgenic mice expressing a mutant rhodopsin gene. Invest Ophthalmol Vis Sci 1995; 36: 62–71.
Goto Y . An electrode to record the mouse cornea electroretinogram. Doc Ophthalmol 1996; 91: 147–154.
Acknowledgements
We thank H Fujii, Y Hori, and R Hashimoto for assistance with the experiments. Mr Brian Quinn provided language assistance. This work was supported by a Grant of Promotion of Basic Science Research in Medical Frontier of the Organization for Pharmaceutical Safety and Research.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ikeda, Y., Goto, Y., Yonemitsu, Y. et al. Simian immunodeficiency virus-based lentivirus vector for retinal gene transfer: a preclinical safety study in adult rats. Gene Ther 10, 1161–1169 (2003). https://doi.org/10.1038/sj.gt.3301973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301973
Keywords
This article is cited by
-
Ocular gene delivery using lentiviral vectors
Gene Therapy (2012)
-
Sendai viral vector mediated angiopoietin-1 gene transfer for experimental ischemic limb disease
Angiogenesis (2009)
-
MicroRNA-133 controls cardiac hypertrophy
Nature Medicine (2007)
-
Lentiviral vectors
Journal of Biomedical Science (2004)